��Ŀ����
2�������������У���HCl����N2����NH3����Na2O2����H2O2����NH4Cl����NaOH����Ar����CO2����C2H4��1��ֻ���ڷǼ��Լ��ķ����Ǣڣ��ȴ��ڷǼ��Լ��ִ��ڼ��Լ��ķ����Ǣݢ⣻ֻ���ڼ��Լ��ķ����Ǣ٢ۢᣮ
��2��ֻ���ڵ����ķ����Ǣ٢ۢݣ����������ķ����Ǣڣ�ֻ����˫���ķ����Ǣᣬ�ȴ��ڵ����ִ���˫���ķ����Ǣ⣮
��3��ֻ���ڦҼ��ķ����Ǣ٢ۢݣ��ȴ��ڦҼ��ִ��ڦм��ķ����Ǣڢ�⣮
��4�������ڻ�ѧ�����Ǣ࣮
��5���ȴ������Ӽ��ִ��ڼ��Լ����Ǣޢߣ��ȴ������Ӽ��ִ��ڷǼ��Լ����Ǣܣ���������ţ�
���� һ����˵�����ý����ͻ��÷ǽ���Ԫ��֮�����γ����Ӽ���ͬ�ַǽ���Ԫ��֮�����γɷǼ��Թ��ۼ�����ͬ�ǽ���Ԫ��֮�����γɼ��Թ��ۼ���笠����Ӻ��������֮��������Ӽ������йµ��ӶԺͺ��пչ����ԭ��֮�������λ�����ݴ˷������
��� �⣺��1��ֻ���ڷǼ��Լ�����ͬԭ���γɵĻ�ѧ�����Ǽ����ķ�����N2���ȴ��ڷǼ��Լ��ִ��ڼ��Լ��ķ����ǣ�H2O2��C2H4��ֻ���ڼ��Լ��ķ����ǣ�HCl��NH3��CO2���ʴ�Ϊ���ڣ��ݢ⣻�٢ۢ
��2��ֻ���ڵ����ķ����ǣ���HCl����NH3����H2O2�����������ķ����Ǣ�N2��ֻ����˫���ķ����Ǣ�CO2���ȴ��ڵ����ִ���˫���ķ����Ǣ�C2H4��
�ʴ�Ϊ���٢ۢݣ��ڣ���⣻
��3��ֻ���ڵ����ķ��Ӷ����ڦҼ��ķ����ǣ���HCl����NH3����H2O2���ȴ��ڦҼ��ִ��ڦм��ķ��ӣ����ӱ��뺬��˫�����������Ǣ�N2����CO2����C2H4��
�ʴ�Ϊ���٢ۢݣ��ڢ�⣻
��4�������ڻ�ѧ������ϡ�����壬�ʴ�Ϊ���ࣻ
��5���ȴ������Ӽ��ִ��ڼ��Լ����Ǣ�NH4Cl����NaOH���ȴ������Ӽ��ִ��ڷǼ��Լ����Ǣ�Na2O2���ʴ�Ϊ���ޢߣ��ܣ�
���� ���⿼�����Ӽ������ۼ�����λ����֪ʶ�㣬���ؿ�����������ȷ������ں��ǽⱾ��ؼ���ע����λ�����ڹ��ۼ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | H3��Ħ��������3g | |
| B�� | H3����Ԫ�ص�һ���µ�ͬλ�� | |
| C�� | H3�������3������ | |
| D�� | H2��H3����Ԫ���γɵ����ֲ�ͬ���� |
| A�� | ��ϡHNO3�еμ�Na2SO3��Һ��SO32-+2H+�TSO2��+H2O | |
| B�� | ��Na2SiO3��Һ��ͨ�����SO2��SiO32-+SO2+H2O�TH2SiO3��+SO32- | |
| C�� | ��Al2��SO4��3��Һ�м��������NH3•H2O��Al3++4NH3•H2O�TAlO2-+4NH4++2H2O | |
| D�� | ��CuSO4��Һ�м���Na2O2��2Na2O2+2Cu2++2H2O�T4Na++2Cu��OH��2��+O2�� |
| A�� | ��84������Һ�У�K+��CO32-��Na+��I- | |
| B�� | $\frac{K_W}{{C��{H^+}��}}$=1��10-13mol��L-1����Һ�У�NH4+��Ca2+��Cl-��NO3- | |
| C�� | ��ʹPH��ֽ����ɫ����Һ�У�Na+��CH3COO-��Fe3+��SO42- | |
| D�� | ͨ��������H2S�����Һ�У�Al3+��Cu2+��SO42-��Cl- |
| A�� | 60��ʱ��NaCl��Һ��pH��7������Һ������ | |
| B�� | pH=4.5�ķ���֭��c��H+����pH=6.5��ţ����c��H+����100�� | |
| C�� | �к�pH���������ͬ������ʹ�����Һ������NaOH�����ʵ�����ͬ | |
| D�� | ��ͬ�¶��£�1 mol•L-1��ˮ��0.5 mol•L-1��ˮ��c��OH-��֮����2��1 |
| A�� | ���³�ѹ�£�46g NO2��N2O4�Ļ�����庬��ԭ������Ϊ3NA | |
| B�� | 92g�ױ��к���̼̼˫����ĿΪ3 NA | |
| C�� | 1 L 1 mol•L-1��Na2CO3��Һ�к���CO32-����ĿΪNA | |
| D�� | ��״���£�2.24L Cl2��ˮ��Ӧת�Ƶĵ�����ĿΪ0.1NA |

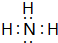 ����
����
 ����
���� �������ȣ�ClO2������ɫ������ˮ�����壩�Ǹ�Ч���Ͷ������������ش��������⣺
�������ȣ�ClO2������ɫ������ˮ�����壩�Ǹ�Ч���Ͷ������������ش��������⣺ ��1mol O22+�к��еĦм���ĿΪ2NA��
��1mol O22+�к��еĦм���ĿΪ2NA��