��Ŀ����
9�� A��B��C�Ƕ����ڷǽ���Ԫ�أ��˵������������AԪ��ԭ�Ӻ��������ֲ�ͬ���ܼ��Ҹ����ܼ������ĵ�������ͬ��C�ǵؿ��к�������Ԫ�أ�D��E�ǵ�������Ԫ�أ�����EԪ�صĺ˵����Ϊ29��Dԭ�Ӻ���δ�ɶԵ�������ͬ��������࣮���ö�Ӧ��Ԫ�ط��Ż�ѧʽ��գ�
A��B��C�Ƕ����ڷǽ���Ԫ�أ��˵������������AԪ��ԭ�Ӻ��������ֲ�ͬ���ܼ��Ҹ����ܼ������ĵ�������ͬ��C�ǵؿ��к�������Ԫ�أ�D��E�ǵ�������Ԫ�أ�����EԪ�صĺ˵����Ϊ29��Dԭ�Ӻ���δ�ɶԵ�������ͬ��������࣮���ö�Ӧ��Ԫ�ط��Ż�ѧʽ��գ���1��A��B��C�ĵ�һ��������С�����˳��ΪC��O��N��
�� 2 ��B���⻯����Һ����ԭ����NH3���Ӽ����γ�������е�ߣ�
�� 3 ����֪A��C�γɵĻ�������Ӽ��������ЧӦ��l mol���к��Цм�����ĿΪ1.204��1024����2NA��
��4����̬Dԭ�ӵ���Χ�����Ų�ʽΪ3d54s1��DO2Cl2�۵㣺-96.5�棬�е㣺117�棬���̬DO2Cl2���ڷ��Ӿ��壮
��5��E���⻯��ľ����ṹ��ͼ��ʾ���仯ѧʽ��CuH��
���� A��B��C�Ƕ����ڷǽ���Ԫ�أ��˵������������AԪ��ԭ�Ӻ��������ֲ�ͬ���ܼ��Ҹ����ܼ������ĵ�������ͬ����A��CԪ�أ�C�ǵؿ��к�������Ԫ�أ���C��OԪ�أ�Bԭ����������A��С��C������B��NԪ�أ�
D��E�ǵ�������Ԫ�أ�����EԪ�صĺ˵����Ϊ29����E��Cu��Dԭ�Ӻ���δ�ɶԵ�������ͬ��������࣬��D��CrԪ�أ�
��1��ͬһ����Ԫ�أ�Ԫ�ص�һ����������ԭ��������������������ƣ�����IIA�塢��VA��Ԫ�ص�һ�����ܴ���������Ԫ�أ�
��2������������⻯���۷е�ϸߣ�
��3��A��C�γɵĻ�������Ӽ��������ЧӦ����Ϊ������̼��ÿ��O=C=O�����к���2���м���
��4��D��CrԪ�أ���3d��4s�ܼ�����Ϊ����Χ���ӣ����ݹ���ԭ����д����Χ�����Ų�ʽ��
���Ӿ����۷е�ϵͣ�
��5�����þ�̯��ȷ���þ�����ѧʽ��
��� �⣺A��B��C�Ƕ����ڷǽ���Ԫ�أ��˵������������AԪ��ԭ�Ӻ��������ֲ�ͬ���ܼ��Ҹ����ܼ������ĵ�������ͬ����A��CԪ�أ�C�ǵؿ��к�������Ԫ�أ���C��OԪ�أ�Bԭ����������A��С��C������B��NԪ�أ�
D��E�ǵ�������Ԫ�أ�����EԪ�صĺ˵����Ϊ29����E��Cu��Dԭ�Ӻ���δ�ɶԵ�������ͬ��������࣬��D��CrԪ�أ�
��1��ͬһ����Ԫ�أ�Ԫ�ص�һ����������ԭ��������������������ƣ�����IIA�塢��VA��Ԫ�ص�һ�����ܴ���������Ԫ�أ����Ե�һ������C��O��N���ʴ�Ϊ��C��O��N��
��2��B���⻯���ǰ���������������⻯���۷е�ϸߣ������к�������������۷е�ϸߣ�������Һ�����ʴ�Ϊ��NH3���Ӽ����γ�������е�ߣ�
��3��A��C�γɵĻ�������Ӽ��������ЧӦ����Ϊ������̼��ÿ��O=C=O�����к���2���м�����1mol������̼�����Цм�����Ϊ1.204��1024����2NA���ʴ�Ϊ��1.204��1024����2NA��
��4��D��CrԪ�أ���3d��4s�ܼ�����Ϊ����Χ���ӣ����ݹ���ԭ����д����Χ�����Ų�ʽΪ3d54s1��
���Ӿ����۷е�ϵͣ��������۷е�ϵͣ�����Ϊ���Ӿ��壬
�ʴ�Ϊ��3d54s1�����ӣ�
��5������ԭ�Ӱ뾶��С֪��С���ʾHԭ�ӡ������ʾCuԭ�ӣ�Hԭ�Ӹ���=4��Cuԭ�Ӹ���=8��$\frac{1}{8}$+6��$\frac{1}{2}$=4������H��Cuԭ�Ӹ���=4��4=1��1�������仯ѧʽΪCuH��
�ʴ�Ϊ��CuH��
���� ���⿼�����ʽṹ�����ʣ�Ϊ��Ƶ���㣬�漰�������㡢ԭ�Ӻ�������Ų���Ԫ�������ɡ������֪ʶ�㣬��ȷ����ԭ��������ԭ�ӽṹ��Ԫ���������ǽⱾ��ؼ������ؿ���ѧ���������㼰�ռ�������������Ŀ�ѶȲ���


| A�� | ����ʽΪC16H16O9 | |
| B�� | ����̼������Һ��Ӧ����������������̼ | |
| C�� | �ܷ�����������Ӧ�������ܷ���������Ӧ�IJ��� | |
| D�� | NaOH��H2����ˮ�ֱ���1mol��ԭ�ᷴӦʱ�����ķ�Ӧ���������ʵ�����Ϊ4mol |

| A�� | 60 g SiO2�������2��6.02��1023��Si-O�� | |
| B�� | ʯīϩ����̼ԭ�ӹ��ɵĵ���Ƭ״�ṹ���²��ϣ��ṹʾ��ͼ��ͼ�ף�����0.12 gʯīϩ�к���6.02��1022��̼ԭ�� | |
| C�� | 720 g C60���壨��ͼ�ң��к���6.02��1023�������ṹ��Ԫ | |
| D�� | 14 g����ϩ��CnH2n��CmH2m�������й��õ��Ӷ���ĿΪ3��6.02��1023 |
 ��ij��������ʽΪC6H14������������������Ȳ���������ӳɵõ�����������Ľṹ��ʽΪ��CH3��2CHCH2CH2CH3��
��ij��������ʽΪC6H14������������������Ȳ���������ӳɵõ�����������Ľṹ��ʽΪ��CH3��2CHCH2CH2CH3��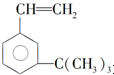 ���������ʣ�
���������ʣ�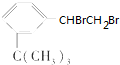 ��
�� ��ͼ��ʵ������ȡijЩ�����װ�ã�
��ͼ��ʵ������ȡijЩ�����װ�ã� CH3+��
CH3+�� CH3-��
CH3-��
 CO+3H2��
CO+3H2��