��Ŀ����
11��A��B��C��D��E��F��G��Ϊ������Ԫ�أ�ԭ���������ε�����AԪ��ԭ�Ӻ��������ӣ�BԪ��ԭ�������������Ǵ�����������2����D�ǵؿ��к�������Ԫ�أ�E�Ƕ������н�������ǿ��Ԫ�أ�F��Gλ�����ڣ�G��ͬ����Ԫ����ԭ�Ӱ뾶��С��Ԫ�أ����û�ѧ����ش���1���ƶ�B��Ԫ�����ڱ��е�λ�õڶ����ڢ�A�壬д��E2D�ĵ���ʽ
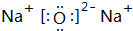 ��
����2��д��A��D�γɵ�10���������ӵĻ�ѧʽH3O+��
��3��E��F��G����Ԫ�����γɵļ����ӣ��뾶�ɴ�С��˳����S2-��Cl-��Na+��
��4�������£�1molA�ĵ�����D�ĵ�������ȫȼ������Һ̬ˮ���ų�286kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽH2��g��+$\frac{1}{2}$O2��g���TH2O��l����H=-286kJ•mol-1��
��5����Fe��Cu�Ļ�����У�����һ������C������������Ӧ��ˮ�����ϡ��Һ����ַ�Ӧ��ʣ�����m1g���������м���һ������ϡ���ᣬ��ַ�Ӧ��ʣ�����m2g������˵����ȷ����AC��
A��m1һ������m2B��ʣ�����m2��һ��û�е���Cu
C������ϡ����ǰ�������Һ�п϶�����Fe2+D������ϡ����ǰ�������Һ�п϶�����Cu2+��
���� A��B��C��D��E��F��G��Ϊ������Ԫ�أ�ԭ���������ε�����AԪ��ԭ�Ӻ��������ӣ���AΪ��Ԫ�أ�BԪ��ԭ�Ӻ��������������Ǵ�����������2������B��2�����Ӳ㣬�������4�����ӣ���BΪ̼Ԫ�أ�DԪ���ǵؿ��к�������Ԫ�أ���DΪOԪ�أ�C��ԭ����������̼����֮�䣬��CΪNԪ�أ�EԪ���Ƕ�����Ԫ���н�������ǿ��Ԫ�أ���EΪNa��F��G��λ�����ڣ�G��ͬ����Ԫ����ԭ�Ӱ뾶��С��Ԫ�أ�����֪FΪSԪ�ء�GΪClԪ�أ��ݴ˽��
��� �⣺A��B��C��D��E��F��G��Ϊ������Ԫ�أ�ԭ���������ε�����AԪ��ԭ�Ӻ��������ӣ���AΪ��Ԫ�أ�BԪ��ԭ�Ӻ��������������Ǵ�����������2������B��2�����Ӳ㣬�������4�����ӣ���BΪ̼Ԫ�أ�DԪ���ǵؿ��к�������Ԫ�أ���DΪOԪ�أ�C��ԭ����������̼����֮�䣬��CΪNԪ�أ�EԪ���Ƕ�����Ԫ���н�������ǿ��Ԫ�أ���EΪNa��F��G��λ�����ڣ�G��ͬ����Ԫ����ԭ�Ӱ뾶��С��Ԫ�أ�����֪FΪSԪ�ء�GΪClԪ�أ�
��1��BΪ̼Ԫ�أ���Ԫ�����ڱ��е�λ�ã��ڶ����ڢ�A�壬Na2O�ĵ���ʽΪ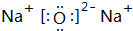 ��
��
�ʴ�Ϊ���ڶ����ڢ�A�壻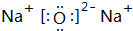 ��
��
��2��HԪ����OԪ���γɵ�10���������ӵĻ�ѧʽΪH3O+���ʴ�Ϊ��H3O+��
��3��Na��S��Cl�γɵ�����ΪNa+��S2-��Cl-�����Ӳ���Խ�����Ӱ뾶Խ���Ӳ�����ͬʱ���˵����Խ�����Ӱ뾶ԽС���ʼ����Ӱ뾶�ɴ�С��˳��ΪS2-��Cl-��Na+��
�ʴ�Ϊ��S2-��Cl-��Na+��
��4�������£�1mol��������������ȫȼ������Һ̬ˮ���ų�286kJ���������÷�Ӧ���Ȼ�ѧ����ʽΪ��H2��g��+$\frac{1}{2}$O2��g���TH2O��l����H=-286kJ•mol-1��
�ʴ�Ϊ��H2��g��+$\frac{1}{2}$O2��g���TH2O��l����H=-286kJ•mol-1��
��5��HNO3����ǿ�����ԣ��ܽ�Fe��Cu����ΪFe3+��Cu2+��Fe3+���н�ǿ�������ԣ��ɽ�Fe��Cu��������һ��ʣ���������ʣ�����ΪFe��Cu����Һ�к��е�����ΪFe2+����ʣ��Cu����Һ��������ΪFe2+�����ܺ���Cu2+��������Һ�к���NO3-�������������£�ʣ��Ľ�����������NO3-��Ӧ�������н���ʣ�࣬����Һ��һ������Fe2+�����ܺ���Cu2+��
A��������������֪��m1һ������m2����A��ȷ��
B��ʣ�����m2��һ�����е���Cu����B����
C������ϡ����ǰ�������Һ�п϶�����Fe2+����C��ȷ��
D������ϡ����ǰ����Һ�в�һ����Cu2+����D����
��ѡ��AC��
���� ���⿼��ṹ����λ�ù�ϵӦ�ã��漰����ʽ�����Ӱ뾶�Ƚϡ��Ȼ�ѧ����ʽ��Ԫ�ػ��������ʵȣ����ض�֪ʶ��������Ǩ��Ӧ���������飬��5����ע���������������������ǿ�����ԣ��Ѷ��еȣ�

 ABC����ȫ�ž�ϵ�д�
ABC����ȫ�ž�ϵ�д�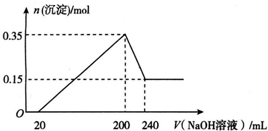 ��һ��������þ���������Ͷ��200 mLϡ�����У�����ȫ���ܽ����������Һ�м���NaOH��Һ�����ɳ��������ʵ���������NaOH��Һ����ı仯��ϵ��ͼ��ʾ��������˵��������ǣ�������
��һ��������þ���������Ͷ��200 mLϡ�����У�����ȫ���ܽ����������Һ�м���NaOH��Һ�����ɳ��������ʵ���������NaOH��Һ����ı仯��ϵ��ͼ��ʾ��������˵��������ǣ�������| A�� | þ������������Ϊ9 g | |
| B�� | ���20 mL NaOH��Һ�����к�����ϡ���� | |
| C�� | ����������Һ�����ʵ���Ũ��Ϊ5 mol•L-1 | |
| D�� | ���ɵ������ڱ�״���µ����Ϊ11.2 L |
| ��Ŀ | �۵�/��C | �ܶ�/ ��g•cm-3�� | Ӳ�ȣ���� ʯΪ10�� | ������ ����Ϊ100�� |
| ij�Ͻ� | 2 500 | 3.00 | 7.4 | 2.3 |
| �� | 1 535 | 7.86 | 4.5 | 17 |
| A�� | ���� | B�� | �Ŵ��� | C�� | ¯�� | D�� | �ɻ���� |

| A�� | Al��Cu��Mg��Si | B�� | Al��Mg��Si��Zn | C�� | Al��Fe��C��Cu | D�� | Al��Si��Zn��Na |
����NaOH��ˮ��Һ���ȡ� ����NaOH�Ĵ���Һ���ȡ� ����Ũ���Ṳ�ȵ�170��
��һ���������������ӳɡ�����Cu��Ag���ڵ���������������� �������Ƶ�Cu��OH��2���ȣ�
| A�� | �ڢܢ٢ݢ� | B�� | �ڢܢ٢ޢ� | C�� | �٢ۢܢڢ� | D�� | �٢ۢܢڢ� |
��H=-57.3kJ/mol����1L 1mol•L-1��������Һ�зֱ����1L 0.5mol•L-1��Ba��OH��2��Һ����ϡ�����ϡ�����ϡ���ᣬ��ȫ��Ӧ����ЧӦ�ֱ�Ϊ��H1����H2����H3�����ǵĹ�ϵ��ȷ���ǣ�������
| A�� | ��H1����H2����H3 | B�� | ��H1����H3����H2 | C�� | ��H2����H3����H1 | D�� | ��H1=��H3����H2 |

| A�� | ���������ǿ�ҵĸ�ʴ�ԣ�Ӧ����Σ�ջ�ѧ��Ʒ�������Ʊ��� | |
| B�� | ȡ10mL���������ձ��У��ټ�18.4mL��ˮ�����9%������ | |
| C�� | ����200mL 4.6 mol/L��ϡ������ȡ������50mL | |
| D�� | ���������������ˮ���������Һ�����ʵ���Ũ��С��9.2mol/L |
 ��W��һ�����뷽��ʽΪH2O2?H++HO2-��
��W��һ�����뷽��ʽΪH2O2?H++HO2-�� ��
��