��Ŀ����
15��ijˮ��Һֻ���ܺ���K+��Al3+��Fe3+��Mg2+��Ba2+��NH4+��Cl-��SO42-�е����������ӣ�ijͬѧȡ100mL����Һ�ֳ����ȷݽ�������ʵ�飺�ٵ�һ�ݼӹ���������������Һ����ȣ��ռ���0.02mol�����壬����������ͬʱ�õ���Һ�ף�
������Һ����ͨ�����Ķ�����̼���壬���ɰ�ɫ���������������ˣ�ϴ�����պõ�1.02g���壮
�۵ڶ��ݼ��������Ȼ�����Һ�����ɰ�ɫ������������������ϴ�ӣ�����õ�11.65g���壮
�ݴˣ���ͬѧ�õ��Ľ�����ȷ���ǣ�������
| A�� | ʵ����в���������Ϊ���������ɵ�ԭ��Һ��c��NH4+��=0.1 mol•L-1 | |
| B�� | ʵ����еij�����һ����BaSO4��������Mg��OH��2 | |
| C�� | ��Ҫ�ж�ԭ��Һ���Ƿ���Cl-�������������ʵ����֤ | |
| D�� | ԭ��Һ��һ����K+����c��K+����0.4 mol•L-1 |
���� �ٵ�һ�ݼӹ���NaOH��Һ����ȣ��ռ���0.02mol���壬��Ϊ������һ������NH4+�����ʵ���Ϊ0.02mol��Ũ��Ϊ��$\frac{0.02mol}{0.05L}$=0.4mol/L���������ɣ���һ��������Fe3+��Mg2+��
�������Һ��ͨ�����CO2�����ɰ�ɫ��������Ϊ������������ԭ��Һ��һ����Al3+��һ��������̼������ӣ������Ӻ������������Ʒ�Ӧ����ƫ��������Һ����Һ��ͨ�����CO2�����ɰ�ɫ��������Ϊ���������������������������ˡ�ϴ�ӡ����պõ�1.02g���弴Ϊ��������������Ԫ���غ㣬�õ������ӵ����ʵ�����$\frac{1.02g}{102g/mol}$��2=0.02mol��Ũ��Ϊ��$\frac{0.02mol}{0.05L}$=0.4mol/L��
�۵ڶ��ݼ�����BaCl2��Һ�����ɰ�ɫ��������һ��������������ӣ��ޱ����ӣ���������������ϴ�ӡ�����õ�11.65g���弴���ᱵ��������11.65g�����ʵ���Ϊ��$\frac{11.65g}{233g/mol}$=0.05mol������Ԫ���غ㣬������������ӵ����ʵ�����0.05mol��Ũ��Ϊ��$\frac{0.05mol}{0.05L}$=1mol/L��
���Ͽ�֪��һ�����е������ǣ�NH4+��Al3+��SO42-����Ũ�ȷֱ��ǣ�0.4mol/L��0.4mol/L��1mol/L��һ������Fe3+��Mg2+��Ba2+��SO42������ȷ���Ƿ���������ӣ��Դ˽����⣮
��� �⣺�ٵ�һ�ݼӹ���NaOH��Һ����ȣ��ռ���0.02mol���壬��Ϊ������һ������NH4+�����ʵ���Ϊ0.02mol��Ũ��Ϊ��$\frac{0.02mol}{0.05L}$=0.4mol/L���������ɣ���һ��������Fe3+��Mg2+��
�������Һ��ͨ�����CO2�����ɰ�ɫ��������Ϊ������������ԭ��Һ��һ����Al3+��һ��������̼������ӣ������Ӻ������������Ʒ�Ӧ����ƫ��������Һ����Һ��ͨ�����CO2�����ɰ�ɫ��������Ϊ���������������������������ˡ�ϴ�ӡ����պõ�1.02g���弴Ϊ��������������Ԫ���غ㣬�õ������ӵ����ʵ�����$\frac{1.02g}{102g/mol}$��2=0.02mol��Ũ��Ϊ��$\frac{0.02mol}{0.05L}$=0.4mol/L��
�۵ڶ��ݼ�����BaCl2��Һ�����ɰ�ɫ��������һ��������������ӣ��ޱ����ӣ���������������ϴ�ӡ�����õ�11.65g���弴���ᱵ��������11.65g�����ʵ���Ϊ��$\frac{11.65g}{233g/mol}$=0.05mol������Ԫ���غ㣬������������ӵ����ʵ�����0.05mol��Ũ��Ϊ��$\frac{0.05mol}{0.05L}$=1mol/L��
���Ͽ�֪��һ�����е������ǣ�NH4+��Al3+��SO42-����Ũ�ȷֱ��ǣ�0.4mol/L��0.4mol/L��1mol/L��һ������Fe3+��Mg2+��Ba2+��SO42������ȷ���Ƿ���������ӣ�
A���������Ϸ�����֪��c ��NH4+��=0.4 mol•L-1����A����
B���������Ϸ�����֪�����еİ�ɫ������һ����BaSO4������Һ������þ���ӣ���û������������þ����B����
C�������Ϸ�����֪������ȷ�����������ӣ���C����
D���κ���Һ�ж����ڵ���غ㣬NH4+��Al3+��SO42-����Ũ�ȷֱ��ǣ�0.4mol/L��0.4mol/L��1mol/L������֪��NH4+��Al3+�����������С��SO42-��������������ݵ���غ㣬��һ����K+���ڣ����������Ӵ��ڣ���0.4��1+0.4��3+c��K+����1=1��2�����c��K+��=0.4mol/L����D��ȷ��
��ѡD��
���� ���⿼�����ӵļ��飬Ϊ��Ƶ���㣬������ѧ���ķ��������������Ŀ��飬��Ŀ���ö���ʵ��Ͷ�������������ϵ�ģʽ�������˽����Ѷȣ�ͬʱ�漰���ӹ��桢���ӷ�Ӧ�ȶ��ǽ�����ע�����Ϣ��������K+��ȷ���׳���ʧ��

| A�� | ��ɫ��Һ��Cu2+��H+��Cl-��HSO3- | |
| B�� | ��ʹpH��ֽ�ʺ�ɫ����Һ��Na+��Fe2+��Cl-��NO3- | |
| C�� | ��ˮ�������c��H+��=1��10-12mol/L����Һ�У�K+��Fe3+��Cl-��NO3- | |
| D�� | $\frac{{K}_{w}}{c��{H}^{+}��}$=0.1 mol/L����Һ��Na+��K+��SiO32-��NO3- |
��1���������ӵĽṹʾ��ͼΪ
 ��
���ڼ���ʱ����Ԫ�ص�����������Ӧˮ�����Ũ��Һ��ľ̿��Ӧ�Ļ�ѧ����ʽΪC+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$2SO2��+CO2��+2H2O��
��2��25�棬������ĵ���ƽ�ⳣ�����±���
| Ka1 | Ka2 | |
| H2SO3 | 1.3��10-2 | 6.3��10-8 |
| H2CO3 | 4.2��10-7 | 5.6��10-11 |
��0.10mol•L-1Na2SO3��Һ������Ũ���ɴ�С��˳��Ϊc��Na+����c��SO32-����c��OH-����c��HSO3-����c��H+����
��H2SO3��Һ��NaHCO3��Һ��Ӧ�����ӷ���ʽΪH2SO3+HCO3-=HSO3-+CO2��+H2O��
| A�� | NaB��Һ��pH=8��c��Na+��-c��B-��=9.9��10-7 mol/L | |
| B�� | Na2CO3��Һ�У�2c��Na+��=c��CO${\;}_{3}^{2-}$��+c��HCO${\;}_{3}^{-}$��+c��H2CO3�� | |
| C�� | pH��ȵĢ�NH4NO3���ڣ�NH4��2SO4����NH4HSO4������Һ�У�c��NH${\;}_{4}^{+}$����С˳��Ϊ���٣��ڣ��� | |
| D�� | 10 mL pH=12������������Һ�м���pH=2��HA��Һ��pH�պõ���7����������Һ���V���ܣ�=20 mL |
 ���������ձ�����һ����Ҫ���ָ����SO2�ĺ����������ѧ֪ʶ�ش��������⣮��ҵ������Ĺ����У�SO2��������ԭ��Ϊ��
���������ձ�����һ����Ҫ���ָ����SO2�ĺ����������ѧ֪ʶ�ش��������⣮��ҵ������Ĺ����У�SO2��������ԭ��Ϊ��2SO2��g��+O2��g��?������ 2SO3��g��+Q
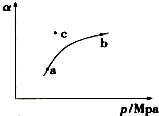
ij�¶��£����SO2��g����ƽ��ת���ʣ�a������ϵ��ѹǿ�� p ���Ĺ�ϵ��ͼ��ʾ��
��1��a��b�����Ӧ��ƽ�ⳣ��K��a��= K��b�� �����������������=������ͬ����SO3Ũ��c��a����c��b����c��ʱ����Ӧ���ʦԣ��������ԣ��棩����һ������SO2��g����O2��g���ֱ�ͨ�뵽���Ϊ2L�ĺ����ܱ������У��ڲ�ͬ�¶��½��з�Ӧ�õ�����е��������ݣ�
| ʵ���� | �¶�/�� | ��ʼ��/mol | ƽ����/mol | ||
| SO2 | O2 | SO2 | O2 | ||
| 1 | T1 | 4 | 2 | x | 0.8 |
| 2 | T2 | 4 | 2 | 0.4 | y |
��3����ȡSO2��β����NaOH��Һ���գ��ɵõ�Na2SO3��NaHSO3�����Σ�
��0.1mol/L��NaHSO3��Һ��c��H+����c��OH-������ˮ��͵������۵ĽǶȽ�����ԭ��HSO3-�ĵ���̶ȴ���ˮ��̶ȣ�������Һ�м��백ˮ�����ԣ���c��Na+��=c��HSO3-��+c��SO32-��+c��H2SO3�� �����������������=������
����0.1mol/L ��Na2SO3��Һ��������NaOH���壬��ȫ�ܽ����Һ��c��Na+����c��SO32-���ı�ֵ�����������С�����ֲ��䡱����
| A�� | 5NH4NO3$\frac{\underline{\;\;��\;\;}}{\;}$2HNO3+4N2��+9H2O�ķ�Ӧ�У�����28 g N2��ת�Ƶĵ�����ĿΪ3.75NA | |
| B�� | �����£�0.2 mol Fe������ˮ������Ӧ�����ɵ�H2������ĿΪ0.3NA | |
| C�� | ����ȼ�ϵ����������22.4 L����״��������ʱ����·��ͨ���ĵ�����ĿΪ4NA | |
| D�� | �����£�1 L pH=13��NaOH��Һ�У���ˮ�����OH-��ĿΪ10-13NA |
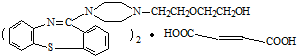 �����ĺϳ�·�����£�
�����ĺϳ�·�����£�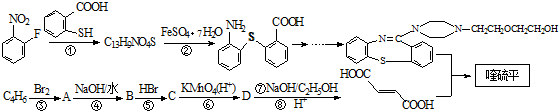
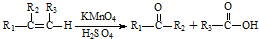
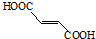 ���еĹ���������Ϊ̼̼˫�����Ȼ���
���еĹ���������Ϊ̼̼˫�����Ȼ���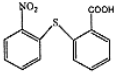 ��
��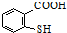 ��һ�������·������۷�Ӧ�Ļ�ѧ����ʽΪ
��һ�������·������۷�Ӧ�Ļ�ѧ����ʽΪ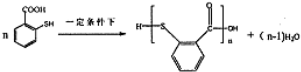 ��
��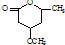 ��һ����Ҫ���л����������ƺ�����������ɴ�
��һ����Ҫ���л����������ƺ�����������ɴ�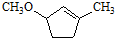 ��
��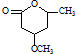 �ĺϳ�·������ͼ�����Լ����ã���
�ĺϳ�·������ͼ�����Լ����ã��� ��ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص������Լ���ǩ�ϵIJ������ݣ�
��ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص������Լ���ǩ�ϵIJ������ݣ�