��Ŀ����
3����Ԫ�ص��⻯���ڹ�ҵ�������������ж��й㷺Ӧ�ã��ش��������⣺��1�������ĽṹʽΪ
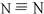 �������⻯����˰���������һ���ڳ����³�Һ̬���£�����ʽΪN2H4����д���µĵ���ʽ
�������⻯����˰���������һ���ڳ����³�Һ̬���£�����ʽΪN2H4����д���µĵ���ʽ ��
����2��NH3��NaClO��Ӧ�ɵõ��£��÷�Ӧ�Ļ�ѧ����ʽΪ2NH3+NaClO=N2H4+NaCl+H2O��
��3�����ڼ��Ի����¿����γ���һ����ȼ�ϵ�أ��±�����ΪN2���õ�ظ����ķ�ӦʽΪN2H4+4OH--4e-=4H2O+N2����ÿ����56g N2Ҫ���ı�״���µ����������Ϊ44.8 L��
���� ��1�����������ǵ�ԭ�Ӻ͵�ԭ�Ӽ��γ��������µķ���ʽΪN2H4���ǵ�ԭ�Ӻ���ԭ���γ��ĸ����ۼ�����ԭ�Ӻ͵�ԭ��֮���γ�һ�����ۼ��γɵĹ��ۻ����
��2��NH3��NaClO��Ӧ�ɵõ��£�N2H4����NԪ�صĻ��ϼ����ߣ��ʻ������Ȼ�����ˮ��
��3������Ϊȼ�ϵ��ʱ����������������Ӧ�ĽǶȿ�֪N2H4����������N2�����ݵ缫��Ӧ�͵����غ���㣮
��� �⣺��1�����������ǵ�ԭ�Ӻ͵�ԭ�Ӽ��γ������������ĽṹʽΪ��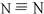 ���µķ���ʽΪN2H4���ǵ�ԭ�Ӻ���ԭ���γ��ĸ����ۼ�����ԭ�Ӻ͵�ԭ��֮���γ�һ�����ۼ��γɵĹ��ۻ��������ʽΪ��
���µķ���ʽΪN2H4���ǵ�ԭ�Ӻ���ԭ���γ��ĸ����ۼ�����ԭ�Ӻ͵�ԭ��֮���γ�һ�����ۼ��γɵĹ��ۻ��������ʽΪ�� ��
��
�ʴ�Ϊ��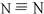 ��
�� ��
��
��2��NH3��NaClO����������ԭ��Ӧ�ɵõ��£�N2H4�����Ȼ��ƺ�ˮ�����Ը÷�Ӧ�Ļ�ѧ����ʽΪ��2NH3+NaClO=N2H4+NaCl+H2O��
�ʴ�Ϊ��2NH3+NaClO=N2H4+NaCl+H2O��
��3����һ����ȼ�ϼ��Ե���У���������ʧ���Ӻ����������ӷ�Ӧ����ˮ�͵������缫��ӦʽΪ��N2H4+4OH--4e-=4H2O+N2�������缫��ӦΪ��O2+2H2O+4e-=4OH-��ÿ����56g N2���ʵ���=$\frac{56g}{28g/mol}$=2mol�����������������ʵ���Ϊ2mol�����ݵ����غ�������ı�״���µ����������Ϊ2mol��22.4L/mol=44.8L��
�ʴ�Ϊ��N2H4+4OH--4e-=4H2O+N2����44.8L��
���� ���⿼���˹���ɡ��缫��Ӧʽ����д��֪ʶ�㣬��Щ���Ǹ߿����ȵ㣬ע��缫��Ӧʽ����дҪ��ϵ������Һ������ԣ��Ѷ��еȣ�

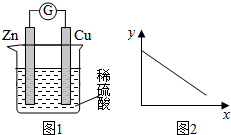
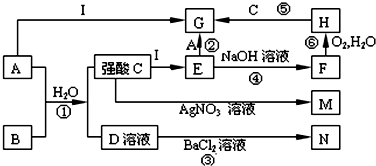
| A�� | ͭ�������� | B�� | c��Zn2+�� | C�� | c��H+�� | D�� | c��SO42-�� |
���̷� ������������ ����ˮ �ܱ��� ���������� �������ƣ�
| A�� | ȫ�� | B�� | �٢ڢۢܢ� | C�� | ֻ�Т٢ڢ� | D�� | ֻ�Тڢۢ� |
| A�� | ͬŨ�ȵ�AOH��Һ��H2B��Һ������̶�ǰ�ߴ��ں��� | |
| B�� | HB-�ĵ���̶ȴ���HB-��ˮ��̶� | |
| C�� | ���εĵ��뷽��ʽΪAHB�TA++H++B2- | |
| D�� | ����Һ������Ũ�ȴ�С˳��һ��Ϊ��c��A+����c��HB-����c��OH-����c��B2-����c��H+�� |
| Ԫ�ر��Ԫ������ | �� | �� | �� | �� | �� | �� | �� | �� |
| ԭ�Ӱ뾶��10-10m�� | 0.74 | 1.60 | 1.52 | 1.10 | 0.99 | 1.86 | 0.75 | 1.43 |
| �����ͻ��ϼ� | +2 | +1 | +5 | +7 | +1 | +5 | +3 | |
| -2 | -3 | -1 | -3 |
��1������Ԫ���д���ͬһ������Тܺ͢ߡ��ۺ͢ޣ����ڵ�3���ڵ��Тڢܢݢޣ����Ͼ��� ��ű�ʾ����
��2��Ԫ�آ���Ԫ�آ���Ƚϣ���̬�⻯����ȶ�����NH3������̬�⻯��Ļ�ѧʽ����
��3��Ԫ�آ١������γ����ֻ����д�����н��ȶ��Ļ�������CO2��Ӧ�Ļ�ѧ����ʽ��2Na2O2+2CO2=Na2CO3+O2��
��4���ϱ���ijԪ������������Ӧ��ˮ����������������������ƣ���Ԫ�صĵ���������������Һ��Ӧ�����ӷ���ʽΪ2Al+2OH-+2H2O=2AlO2-+3H2����
| A�� | �Դ��ʹ�������ǽ��еġ���������������������������Ӧ����ԭ���ԭ�� | |
| B�� | �Ȼ������۵���������ͣ���˹�ҵ����ò��õ�������Ȼ������Ʊ������� | |
| C�� | ����ұ�����ơ��ơ�þ�������������õĽ�������ⷨ������Ψһ���еĹ�ҵ���� | |
| D�� | ���ʱ��ͨ���Ѵ��ƵĽ�����Ʒ���������ѶƲ���������� |
| A�� | ����4.48L | B�� | ��2.24L | C�� | ����2.24L | D�� | ����2.24L |
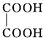
 +NaBr+H2O��
+NaBr+H2O��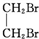 +2NaOH$��_{��}^{ˮ}$
+2NaOH$��_{��}^{ˮ}$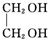 +2NaBr��
+2NaBr��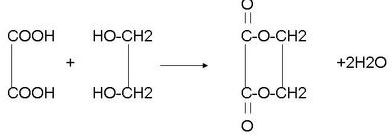 ��
��