��Ŀ����
������������Ч���ٶ���������ŷţ�ʵ�����÷�ú�ң���Ҫ��Al2O3��SiO2�ȣ��Ʊ���ʽ������[Al2��SO4��x��OH��6-2x]��Һ�����������������о���
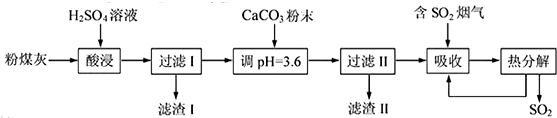
��1�����ʱ��Ӧ�Ļ�ѧ����ʽΪ �����������Ҫ�ɷ�Ϊ ���ѧʽ����
��2����CaCO3������Һ��pH��3.6����Ŀ�����к���Һ�е��ᣬ��ʹAl2��SO4��3ת��ΪAl2��SO4��x��OH��6-2x�����������Ҫ�ɷ�Ϊ ���ѧʽ��������Һ��pHƫ�ߣ����ᵼ����Һ����Ԫ�صĺ������ͣ���ԭ���� �������ӷ���ʽ��ʾ����
��3�����������о���ȫ�ȷֽ�ų���SO2������С�����յ�SO2����������Ҫԭ���� ��������SO2ǰ����Һ��ȣ��ȷֽ��ѭ�����õ���Һ��pH�� �����������С�����䡱����

��1�����ʱ��Ӧ�Ļ�ѧ����ʽΪ
��2����CaCO3������Һ��pH��3.6����Ŀ�����к���Һ�е��ᣬ��ʹAl2��SO4��3ת��ΪAl2��SO4��x��OH��6-2x�����������Ҫ�ɷ�Ϊ
��3�����������о���ȫ�ȷֽ�ų���SO2������С�����յ�SO2����������Ҫԭ����
���㣺���⼯��,�����������Ⱦ������
ר�⣺ʵ�������,Ԫ�ؼ��仯����
��������ú�Һ�ϡ�����ϣ�������ӦAl2O3+3H2SO4�TAl2��SO4��3+3H2O��SiO2��ϡ�����Ӧ��������Һ��������ΪSiO2����Һ�к���Al2��SO4��3������pH=3.6������CaCO3��ĩ��������ӦCaCO3+2H+�TCa2++CO2��+H2O��CaSO4Ϊ�������������ijɷ���ҪΪCaSO4�����˵���Һ���������ˮ��Ӧ���ɵ�SO32-�ױ���������SO42-�����������ת��Ϊǿ������ӣ��ٽ����Ŀ�������
���
�⣺��ú�Һ�ϡ�����ϣ�������ӦAl2O3+3H2SO4�TAl2��SO4��3+3H2O��SiO2��ϡ�����Ӧ��������Һ��������ΪSiO2����Һ�к���Al2��SO4��3������pH=3.6������CaCO3��ĩ��������ӦCaCO3+2H+�TCa2++CO2��+H2O��CaSO4Ϊ�������������ijɷ���ҪΪCaSO4�����˵���Һ���������ˮ��Ӧ���ɵ�SO32-�ױ���������SO42-��
��1��ͨ�����Ϸ���֪�����ʱ��Ӧ�Ļ�ѧ����ʽΪAl2O3+3H2SO4�TAl2��SO4��3+3H2O����������ϡ������ȫ��Ӧ�����������ϡ�����Ӧ����������I�ijɷ�ΪSiO2��
�ʴ�Ϊ��Al2O3+3H2SO4=Al2��SO4��3+3H2O��SiO2��
��2��ͨ�����Ϸ���֪��������ijɷ���CaSO4������Һ��pHƫ�ߣ���Һ�е�Al 3+��OH-���ӷ�Ӧ����Al��OH��3�����Խ��ᵼ����Һ����Ԫ�صĺ������ͣ���Ӧ����ʽΪ3CaCO3+2Al3++3SO42-+3H2O�T2Al��OH��3+3CaSO4+3CO2����
�ʴ�Ϊ��CaSO4��3CaCO3+2Al3++3SO42-+3H2O�T2Al��OH��3+3CaSO4+3CO2����
��3�������������պ�����SO32-��SO32-���ȶ����ױ���������SO42-�����������о���ȫ�ȷֽ�ų���SO2������С�����յ�SO2���������ȷֽ�����Һ�����������Ũ�����ٽ�����Al2��SO4��x��OH��6-2x������Һ��������ǿ����Һ��pH��С��
�ʴ�Ϊ����Һ�еIJ���SO32-����������SO42-����С��
��1��ͨ�����Ϸ���֪�����ʱ��Ӧ�Ļ�ѧ����ʽΪAl2O3+3H2SO4�TAl2��SO4��3+3H2O����������ϡ������ȫ��Ӧ�����������ϡ�����Ӧ����������I�ijɷ�ΪSiO2��
�ʴ�Ϊ��Al2O3+3H2SO4=Al2��SO4��3+3H2O��SiO2��
��2��ͨ�����Ϸ���֪��������ijɷ���CaSO4������Һ��pHƫ�ߣ���Һ�е�Al 3+��OH-���ӷ�Ӧ����Al��OH��3�����Խ��ᵼ����Һ����Ԫ�صĺ������ͣ���Ӧ����ʽΪ3CaCO3+2Al3++3SO42-+3H2O�T2Al��OH��3+3CaSO4+3CO2����
�ʴ�Ϊ��CaSO4��3CaCO3+2Al3++3SO42-+3H2O�T2Al��OH��3+3CaSO4+3CO2����
��3�������������պ�����SO32-��SO32-���ȶ����ױ���������SO42-�����������о���ȫ�ȷֽ�ų���SO2������С�����յ�SO2���������ȷֽ�����Һ�����������Ũ�����ٽ�����Al2��SO4��x��OH��6-2x������Һ��������ǿ����Һ��pH��С��
�ʴ�Ϊ����Һ�еIJ���SO32-����������SO42-����С��
���������⿼�������ʵ��Ʊ�ԭ������ȷ���ʵ������ǽⱾ��ؼ�����������Ϸ���ÿһ�������ķ�Ӧ������������֪���������ʵ���;����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д�
ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д� Сѧ������ҵϵ�д�
Сѧ������ҵϵ�д� ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�
�����Ŀ
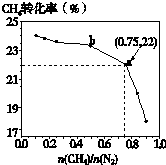 ��֪��3CH4��g��+2N2��g��
��֪��3CH4��g��+2N2��g��| 700�� |
| ���� |
| n(CH4) |
| n(N2) |
A��
| ||
B��
| ||
| C��b���Ӧ��ƽ�ⳣ����a��Ĵ� | ||
| D��a���Ӧ��NH3���������ԼΪ26% |
����������Ԫ��X��Y��Z��W��ԭ��������������Xԭ�Ӻ��������������Ǵ�����2����Y�ķ�����YF3�����и�ԭ�Ӿ��ﵽ8�����ȶ��ṹ��Z��ͬ������ԭ�Ӱ뾶����Ԫ�أ�W���������Ϊ+7�ۣ�����˵����ȷ���ǣ�������
| A��XH4�ķе��YH3�� |
| B��X��W�γɵĻ������Z��W�γɵĻ�����Ļ�ѧ��������ͬ |
| C��Ԫ��Y��W�γɵĻ���������ˮ�����Ư���� |
| D��X��Y�γɵĻ�������������Ӿ��� |
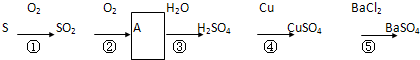
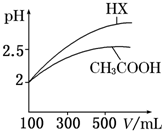 �ش��������⣺
�ش��������⣺