��Ŀ����
����ˮ������Ũ��ˮ��Դ������������ۺ����ú�ˮ����Ҫ;��֮һ��һ�����Ƚ���ˮ������õ�ˮ���ٴ�ʣ���Ũ��ˮ��ͨ��һϵ�й���������ȡ������Ʒ��
�ش��������⣺
��1�����иĽ����Ż���ˮ�ۺ����ù��յ�������������е��� ��������ţ�
���û�������ȡ��ˮ ����߲��ֲ�Ʒ������
���Ż���ȡ��Ʒ��Ʒ�� �ܸĽ��ء��塢þ�ȵ���ȡ����
��2�����á���������������Ũ��ˮ����Br2�����ô������գ������������Ҫ��Ӧ��Br2+Na2CO3+H2O��NaBr+NaBrO3+NaHCO3������1mol Br2ʱ��ת�Ƶĵ�����Ϊ mol��
��3����ˮ��þ��һ�ι���������ͼ��
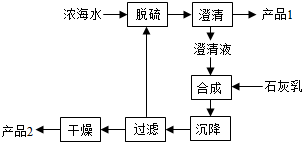
Ũ��ˮ����Ҫ�ɷ����£�
�ù��չ����У��������Ҫ��Ӧ�����ӷ���ʽΪ ����Ʒ2�Ļ�ѧʽΪ ��1LŨ��ˮ���ɵõ���Ʒ2������Ϊ g��
��4������ʯī���������������������ڵ��Ȼ�þ��������Ӧ�Ļ�ѧ����ʽΪ �����ʱ����������ˮ���ڻ���ɲ�Ʒþ�����ģ�д���йط�Ӧ�Ļ�ѧ����ʽ ��
�ش��������⣺
��1�����иĽ����Ż���ˮ�ۺ����ù��յ�������������е���
���û�������ȡ��ˮ ����߲��ֲ�Ʒ������
���Ż���ȡ��Ʒ��Ʒ�� �ܸĽ��ء��塢þ�ȵ���ȡ����
��2�����á���������������Ũ��ˮ����Br2�����ô������գ������������Ҫ��Ӧ��Br2+Na2CO3+H2O��NaBr+NaBrO3+NaHCO3������1mol Br2ʱ��ת�Ƶĵ�����Ϊ
��3����ˮ��þ��һ�ι���������ͼ��

Ũ��ˮ����Ҫ�ɷ����£�
| ���� | Na+ | Mg2+ | Cl- | SO42- |
| Ũ��/��g?L-1�� | 63.7 | 28.8 | 144.6 | 46.4 |
��4������ʯī���������������������ڵ��Ȼ�þ��������Ӧ�Ļ�ѧ����ʽΪ
���㣺���⼯��,��ˮ��Դ�����ۺ�����
ר�⣺±��Ԫ��,��ѧӦ��
��������1��������ʹ���������ʺͽ���ۼ��ɽϴ�ŵĿ�����������Ȼ����˳�ȥ�����ܽ��к�ˮ������ͨ���ı乤�գ�������߲��ֲ�Ʒ���������Ż���ȡ��Ʒ��Ʒ�֣����Ը��ݲ�ͬ��ԭ���Ľ��ء��塢þ�ȵ���ȡ���գ�
��2����Ӧ��Br2������������ԭ�����ã����ݵ���ת���غ��֪���������뻹ԭ�����ʵ���֮��Ϊ5��1���ݴ˼��㣻
��3���������̺ϳɲ����м���ʯ���飬���������˺����Һ��������Ӧ���ø����ӳ��������������������Ƴ�������Ʒ1Ϊ����ƣ��ϳɵõ�������þ�������ʹ��˺����IJ�Ʒ2Ϊ������þ������1L��Һ��Mg2+������������Mg2+��Mg��OH��2����������þ��������
��4����������Ȼ�þ�õ�Mg�����������ʱ����������ˮ���ڣ�Mg��ˮ��Ӧ����������þ������������ɲ�Ʒþ�����ģ�
��2����Ӧ��Br2������������ԭ�����ã����ݵ���ת���غ��֪���������뻹ԭ�����ʵ���֮��Ϊ5��1���ݴ˼��㣻
��3���������̺ϳɲ����м���ʯ���飬���������˺����Һ��������Ӧ���ø����ӳ��������������������Ƴ�������Ʒ1Ϊ����ƣ��ϳɵõ�������þ�������ʹ��˺����IJ�Ʒ2Ϊ������þ������1L��Һ��Mg2+������������Mg2+��Mg��OH��2����������þ��������
��4����������Ȼ�þ�õ�Mg�����������ʱ����������ˮ���ڣ�Mg��ˮ��Ӧ����������þ������������ɲ�Ʒþ�����ģ�
���
�⣺��1���ٻ������Ǽ���һ�ֻ��������磺���������εȣ���ʹˮ��ϸС���������ʺͽ���ۼ��ɽϴ�ŵĿ�����������Ȼ����˳�ȥ����ˮ�п��������ʲ��ܳ�ȥ�����ܽ��к�ˮ�������ʴ���
�ڸĽ����գ������ܼ������������룬��ȥ�ֲ�Ʒ�е����ʣ�������߲�Ʒ������������ȷ��
�ۺ�����һ��Զδ��ȫ�����ľ�ѧ��Դ���⣬��ˮ��Ԫ������ܶ࣬�Ľ����տ����Ż���ȡ��Ʒ��Ʒ�֣�����ȷ��
�ܸ��ݲ�ͬ����ȡԭ�����ԸĽ��ء��塢þ�ȵ���ȡ���գ��Ӷ����K��Br2��Mg�ȵ���ȡ����������ȷ��
��ѡ���ڢۢܣ�
��2����Ӧ��Br2������������ԭ�����ã����ݵ���ת���غ��֪��2��n��������Br2��=2��5��n��ԭ����Br2������n��������Br2����n��ԭ����Br2��=5��1��������1mol Br2ʱ��ת�Ƶĵ�����Ϊ1mol��2��
��5=
mol��
�ʴ�Ϊ��
��
��3���������̺ϳɲ����м���ʯ���飬���������˺����Һ����������Ca2+����SO42-����CaSO4���������ӷ���ʽΪ��Ca2++SO42-=CaSO4�������ù��˵ķ����õ���Ʒ1ΪCaSO4����Һ�м���ʯ���飬������ӦΪMg2++2OH-=Mg��OH��2�����ϳ���Ӧ�õ�Mg��OH��2���������ˡ�����IJ�Ʒ2ΪMg��OH��2��
��Һ��m��Mg2+��=1L��28.8g/L=28.8g��
Mg2+��Mg��OH��2
24g 58g
28.8g m[Mg��OH��2]
m[Mg��OH��2]=28.8g��
=69.6g��
�ʴ�Ϊ��Ca2++SO42-=CaSO4����Mg��OH��2��69.6��
��4����������Ȼ�þ�õ�Mg����������ⷴӦ����ʽΪ��MgCl2�����ڣ�
Mg+Cl2�������ʱ����������ˮ���ڣ�Mg��ˮ��Ӧ����������þ������������ɲ�Ʒþ�����ģ���Ӧ����ʽΪ��Mg+2H2O
Mg��OH��2+H2����
�ʴ�Ϊ��MgCl2�����ڣ�
Mg+Cl2����Mg+2H2O
Mg��OH��2+H2����
�ڸĽ����գ������ܼ������������룬��ȥ�ֲ�Ʒ�е����ʣ�������߲�Ʒ������������ȷ��
�ۺ�����һ��Զδ��ȫ�����ľ�ѧ��Դ���⣬��ˮ��Ԫ������ܶ࣬�Ľ����տ����Ż���ȡ��Ʒ��Ʒ�֣�����ȷ��
�ܸ��ݲ�ͬ����ȡԭ�����ԸĽ��ء��塢þ�ȵ���ȡ���գ��Ӷ����K��Br2��Mg�ȵ���ȡ����������ȷ��
��ѡ���ڢۢܣ�
��2����Ӧ��Br2������������ԭ�����ã����ݵ���ת���غ��֪��2��n��������Br2��=2��5��n��ԭ����Br2������n��������Br2����n��ԭ����Br2��=5��1��������1mol Br2ʱ��ת�Ƶĵ�����Ϊ1mol��2��
| 1 |
| 1+5 |
| 5 |
| 3 |
�ʴ�Ϊ��
| 5 |
| 3 |
��3���������̺ϳɲ����м���ʯ���飬���������˺����Һ����������Ca2+����SO42-����CaSO4���������ӷ���ʽΪ��Ca2++SO42-=CaSO4�������ù��˵ķ����õ���Ʒ1ΪCaSO4����Һ�м���ʯ���飬������ӦΪMg2++2OH-=Mg��OH��2�����ϳ���Ӧ�õ�Mg��OH��2���������ˡ�����IJ�Ʒ2ΪMg��OH��2��
��Һ��m��Mg2+��=1L��28.8g/L=28.8g��
Mg2+��Mg��OH��2
24g 58g
28.8g m[Mg��OH��2]
m[Mg��OH��2]=28.8g��
| 58g |
| 24g |
�ʴ�Ϊ��Ca2++SO42-=CaSO4����Mg��OH��2��69.6��
��4����������Ȼ�þ�õ�Mg����������ⷴӦ����ʽΪ��MgCl2�����ڣ�
| ||
| ||
�ʴ�Ϊ��MgCl2�����ڣ�
| ||
| ||
���������⿼�麣ˮ��Դ�������á�������ԭ��Ӧ���㡢���ԭ���ȣ��ǶԻ���֪ʶ���ۺ����ã���Ҫѧ���߱���ʵ�Ļ������Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
���з�Ӧ�����ӷ���ʽ��ȷ���ǣ�������
| A����AgNO3��Һ�еμӰ�ˮ��������Ag++NH3?H2O�TAgOH��+NH4+ |
| B����Mg��OH��2����Һ�еμ�FeCl3��Һ��3Mg��OH��2+2Fe3+�T2Fe��OH��3+3Mg2+ |
| C����Na2S2O3��Һ�м�������ϡ���2S2O32-+4H+�TSO42-+3S��+2H2O |
D��������Һ��ͨ������CO2���壺 +CO2+H2O��2 +CO2+H2O��2 +CO32- +CO32- |
���������У���ȷ���ǣ�������
| A����ӦA2��g��+3B2��g���T2AB3��g����һ���¶������Է����У���÷�Ӧ�ġ�H��0 |
| B����NH3 ͨ���ȵ�CuSO4 ��Һ����ʹCu2+��ԭ��Cu |
| C���ƺ�þ���Ȼ�����Һ�����ᾧ�����Ĺ��嶼��ˮ��������յ���Ӧ��ˮ�� |
| D�������ܽ������ʱ���ܽ�����������Ĵ����йأ�����Խ������Խ�� |
 25��ʱ�������ᣨHA�������Σ�NaA����ɵĻ����Һ����ʼŨ�Ⱦ�Ϊ1mol?L-1����ͼΪ�����Һ��ͨ��HCl��������NaOH����ʱ����ҺpH�����H+��OH-�����ʵ������仯�����ߣ�����˵���У���ȷ���ǣ�������
25��ʱ�������ᣨHA�������Σ�NaA����ɵĻ����Һ����ʼŨ�Ⱦ�Ϊ1mol?L-1����ͼΪ�����Һ��ͨ��HCl��������NaOH����ʱ����ҺpH�����H+��OH-�����ʵ������仯�����ߣ�����˵���У���ȷ���ǣ�������| A����NaOH��HA����ƽ�ⳣ������ |
| B��ͨ��HCl����Һ��������Ũ��֮�ͼ�С |
| C����Һ��ˮ�ĵ���̶�a��b��c |
| D��b����Һ�У�c��HA����c��A-�� |
 �����£�ȡ20mLijŨ�ȵ�HCl��Ϊ����Һ����һ�����ʵ���Ũ�ȵ�NaOH��Һ���еζ�������������NaOH��Һ��Ϻ�����仯���Բ��ƣ����ζ���������Һ��pH�仯����ͼ��ʾ������������ȷ���ǣ�������
�����£�ȡ20mLijŨ�ȵ�HCl��Ϊ����Һ����һ�����ʵ���Ũ�ȵ�NaOH��Һ���еζ�������������NaOH��Һ��Ϻ�����仯���Բ��ƣ����ζ���������Һ��pH�仯����ͼ��ʾ������������ȷ���ǣ�������| A������HCl��Ũ����0.09mol?L-1��NaOH��ҺŨ��Ϊ0.03mol?L-1 |
| B����B�㣬��Һ������Ũ�ȹ�ϵΪ��c��Cl-����c��Na+����c��H+����c��OH-�� |
| C��A��B��C����ˮ�ĵ���̶ȴ�С����Ϊ��A��B��C |
| D���ζ�ǰ����ƿ�ô���Һ��ϴ������HClŨ��ƫ�� |
 ������ͼ��ʾת����ϵ��X����±�أ���˵������ȷ���ǣ�������
������ͼ��ʾת����ϵ��X����±�أ���˵������ȷ���ǣ�������| A��2H��g��+2X��g���T2HX��g����H3��0 |
| B��;��������HX�ķ�Ӧ����;���أ����ԡ�H1=��H2+��H3 |
| C��Cl��Br��I�ķǽ��������μ���������;�������յ������������� |
| D��;��������HCl�ų�������������HBr�Ķ࣬˵��HCl��HBr�ȶ� |
 A��B��C��D��EΪԭ���������������Ԫ�أ�����ֻ��E�����ڶ����ڣ������Ϣ���±���
A��B��C��D��EΪԭ���������������Ԫ�أ�����ֻ��E�����ڶ����ڣ������Ϣ���±��� ��֪N2��g��+3H2��g��?2NH3��g������H=-92.4kJ?mol-1��
��֪N2��g��+3H2��g��?2NH3��g������H=-92.4kJ?mol-1��