��Ŀ����
17���Ȼ���ͭ��һ��Ӧ�ýϹ�Ĵ�������ˮ�⣮�Ե�Ʒλͭ��ɰ����Ҫ�ɷ�CuS��Ϊԭ���Ʊ��Ȼ���ͭ��·�����£�
��1������1�������MnO2����������������
��2�����̲���Mn2+ת��ΪMnCO3�������÷�Ӧ�����ӷ���ʽΪMn2++HCO3-+NH3=MnCO3��+NH4+����Һ��CuSO4ת��ΪCu��NH3��4CO3������Һ�У�
��3�����������õ�CuO���壬�ò����ڼ�ѹ�����½��е�ԭ���Ǽ�ѹ�����������ݳ���
��4��д���ϳɲ����з�����Ӧ�Ļ�ѧ����ʽ2NaCl+Na2SO3+2CuSO4+H2O=2CuCl+2Na2SO4+H2SO4��
��5���й��ս��ϳɲ����NaCl��Na2SO3��ΪNH4Cl�ͣ�NH4��2SO3����ɻ��һ�ֿ���Ϊ���ʵĸ���Ʒ���仯ѧʽΪ��NH4��2SO4[��NH4HSO4����NH4��2SO4��NH4HSO4����NH4��2SO4��H2SO4��]��
��6��ϴ�Ӳ�����������ϴ��������ˮ�Ҵ�ϴ�ӣ�
���� �Ե�Ʒλͭ��ɰ����Ҫ�ɷ�CuS��Ϊԭ���Ʊ��Ȼ���ͭ��·��Ϊ��������1�����У�������Һ�м���������̡���ͭ������������ԭ��Ӧ�������ʣ��������̱���ԭΪ����������������Һ�У�Ȼ���ڳ��̲����У�����������ת��Ϊ̼���̳�������Һ��CuSO4ת��Ϊ[Cu��NH3��4]CO3������Һ�У�����������[Cu��NH3��4]CO3�����ֽⷴӦ�õ�CuO���塢�����������̼�����������ܽ�CuO�õ�CuSO4���ںϳɲ����У��Ȼ��ơ��������ơ�����ͭ��Ӧ�����Ȼ���ͭ�������ƺ����ᣬ�پ������ˡ�ϴ�ӡ�����õ��Ȼ���ͭ��ϴ�ӳ���ʱ��Ϊ����CuCl�ܽ���ʧ�������ܽ�ƽ�⣬��������ϴ�ӣ������Ҵ�ϴ�ӣ��ݴ˽��
��� �⣺��1������1�ͳ��̹���Ϊ��������Һ�м���������̡���ͭ������������ԭ��Ӧ�������ʣ��������̱���ԭΪ����������������Һ�У�Ȼ���ڳ��̲����У�����������ת��Ϊ̼���̳�������֪����Ԫ�ػ��ϼ۽��ͣ����Զ�����������������
�ʴ�Ϊ����������
��2�����ݳ��̹��̿�֪����Һ�м��백����̼����泥�����Һ�еĶ��������ӷ�Ӧ����̼���̺�笠����ӣ�����ʽ��Mn2++HCO3-+NH3=MnCO3��+NH4+��
�ʴ�Ϊ��Mn2++HCO3-+NH3=MnCO3��+NH4+��
��3��������ܽ������ѹǿ��������������Լ�Сѹǿ�����ڰ������ݳ���
�ʴ�Ϊ����ѹ�����������ݳ���
��4���ںϳɲ����У��Ȼ��ơ��������ơ�����ͭ��Ӧ�����Ȼ���ͭ�������ƺ����ᣬ����ʽΪ��2NaCl+Na2SO3+2CuSO4+H2O=2CuCl+2Na2SO4+H2SO4��
�ʴ�Ϊ��2NaCl+Na2SO3+2CuSO4+H2O=2CuCl+2Na2SO4+H2SO4��
��5�����ϳɲ����NaCl��Na2SO3��ΪNH4Cl �ͣ�NH4��2SO3����Һ�к�������李����ᣬ�ɻ��һ�ֿ��������ʵĸ���Ʒ������Ϊ����NH4��2SO4[��NH4HSO4����NH4��2SO4��NH4HSO4����NH4��2SO4��H2SO4��]��
�ʴ�Ϊ����NH4��2SO4[��NH4HSO4����NH4��2SO4��NH4HSO4����NH4��2SO4��H2SO4��]��
��6���Ȼ���ͭ���ڳ����ܽ�ƽ�⣺CuCl��s�� Cu+��aq��+Cl-��aq����ϴ���Ȼ���ͭʱ��Ϊ�����Ȼ���ͭ���ܽ��������Ӧ��������ϴ�ӣ�Ȼ�����Ҵ�ϴ�ӣ�
�ʴ�Ϊ�����ᣮ
���� ����ͨ���Ȼ���ͭ�Ʊ��Ĺ������̿�����ͭ���仯��������ʣ�ȷ���չ��������ǽ���ؼ�����Ŀ�ѶȽϴ�

| A�� | H2S���ڷǵ���� | B�� | NO��CO���������������� | ||
| C�� | H2Sֻ�л�ԭ��û�������� | D�� | H2S��NO��CO�����������Ӧ |
 ���ܱ������н������з�Ӧ��M������+N������?R������+2A�˷�Ӧ��������ͼ��������ΪR�������ٷֺ���������������ȷ���ǣ�������
���ܱ������н������з�Ӧ��M������+N������?R������+2A�˷�Ӧ��������ͼ��������ΪR�������ٷֺ���������������ȷ���ǣ�������| A�� | ����Ӧ���ȣ�A������ | B�� | ����Ӧ���ȣ�A�ǹ��� | ||
| C�� | ����Ӧ���ȣ�A������ | D�� | ����Ӧ���ȣ�A�ǹ����Һ�� |
| A�� | 28 g��ϩ��28g��ϩ�о�����6NA�Թ��õ��Ӷ� | |
| B�� | ��״���£�2.24L�ȷ��к���C-Cl��ĿΪ0.3NA | |
| C�� | ��״���£�560 mL����ͼ�ȩ�Ļ�������У����еĹ��õ��Ӷ���Ϊ0.2NA | |
| D�� | 11.2L��ϩ����Ȳ�Ļ������Cԭ����ΪNA |
| A�� | Mg | B�� | Ca | C�� | Fe | D�� | Zn |
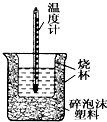 50mL0.05mol•L-1������50mL0.55mol•L-1 NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
50mL0.05mol•L-1������50mL0.55mol•L-1 NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺ ��
�� ��
�� һ���¶��£���2L���ܱ������У�X��Y��Z�������������ʱ��仯��������ͼ��ʾ��
һ���¶��£���2L���ܱ������У�X��Y��Z�������������ʱ��仯��������ͼ��ʾ��