��Ŀ����
ij�л��������ܶ�����ͬ״̬��������38������ȡ7.6g���л����0.1mol O2���ܱ�������ǡ����ȫ��Ӧ������CO2��CO��H2O�����û����������ͨ��Ũ���ᡢ���ȵ�CuO�ͼ�ʯ�Һ���ÿһ������ַ�Ӧ����Ũ��������3.6g��CuO������������1.6g������ʯ������8.8g��
��1���л���ȼ�յ������n��H2O��= mol��n��CO2��= mol��
��2���л���ķ���ʽΪ ��
��3�������ĸ��л���ֱ���������Na��NaOH��Ӧ������Na��NaOH�����ʵ���֮��Ϊ2��1������л���Ľṹ��ʽΪ �����������ŵ�����Ϊ
��4����ͭ������ʱ�����л���ɱ������������䷴Ӧ����ʽΪ
��5�����л���Ҳ�ܺͶ�����C4H10O������������Ӧ���ڶ�����ͬ���칹���У���Щ�ܱ�����������ȩ��д�����з�������Ҫ��Ķ����Ľṹ��ʽ ��
��1���л���ȼ�յ������n��H2O��=
��2���л���ķ���ʽΪ
��3�������ĸ��л���ֱ���������Na��NaOH��Ӧ������Na��NaOH�����ʵ���֮��Ϊ2��1������л���Ľṹ��ʽΪ
��4����ͭ������ʱ�����л���ɱ������������䷴Ӧ����ʽΪ
��5�����л���Ҳ�ܺͶ�����C4H10O������������Ӧ���ڶ�����ͬ���칹���У���Щ�ܱ�����������ȩ��д�����з�������Ҫ��Ķ����Ľṹ��ʽ
���㣺�л���ʵ��ʽ�ͷ���ʽ��ȷ��,�л�����ƶ�
ר�⣺�л���Ļ�ѧ���ʼ��ƶ�,�������������ȼ�չ���
��������1��Ũ���������ˮ�ԣ�Ũ�������������3.6gΪȼ������ˮ��������ͨ����������ͭ�����ڷ�����ӦCuO+CO
Cu+CO2ʹ������������ᣬ���ò������ɼ���CO��������ͨ����ʯ��ʱ����ʯ�ҵ�����������8.8gΪCO2������������ȥCO��CuO��Ӧ���ɵ�CO2������Ϊ�л���ȼ������CO2������������n=
��������ʵ����ʵ�����
��2������Ԫ���غ�����л�����C��H��Oԭ����Ŀ��������û�ѧʽ��
��3�������ĸ��л���ֱ���������Na��NaOH��Ӧ������Na��NaOH�����ʵ���֮��Ϊ2��1��˵�����л��ﺬ��-COOH��-OH���ҷ�����-OH��-COOH��Ŀ��ȣ���Ϸ���ʽ�ݴ���д�ṹ��ʽ��
��4���ǻ��ɱ���������ȩ��
��5�����ǻ�����̼�Ϻ�������ɱ�������ȩ���ݴ�д������Ҫ��Ķ�����
| ||
| m |
| M |
��2������Ԫ���غ�����л�����C��H��Oԭ����Ŀ��������û�ѧʽ��
��3�������ĸ��л���ֱ���������Na��NaOH��Ӧ������Na��NaOH�����ʵ���֮��Ϊ2��1��˵�����л��ﺬ��-COOH��-OH���ҷ�����-OH��-COOH��Ŀ��ȣ���Ϸ���ʽ�ݴ���д�ṹ��ʽ��
��4���ǻ��ɱ���������ȩ��
��5�����ǻ�����̼�Ϻ�������ɱ�������ȩ���ݴ�д������Ҫ��Ķ�����
���
�⣺��1���л���ȼ������ˮ3.6g�������ʵ���=
=0.2mol��
���л���ȼ�����ɵ�COΪx����
CuO+CO
Cu+CO2�������������١�m
28g 16g
x 1.6g
����x=
=2.8g��CO�����ʵ���=
=0.1mol��
����̼Ԫ���غ��֪CO��CuO��Ӧ���ɵ�CO2�����ʵ���Ϊ0.1mol������Ϊ0.1mol��44g/mol=4.4g��
�л���ȼ�����ɵ�CO2������Ϊ8.8g-4.4g=4.4g�����ʵ���Ϊ
=0.1mol��
�ʴ�Ϊ��0.2��0.1��
��2�����л������Է�������Ϊ��38��2=76��7.6g���л�������ʵ���Ϊ��
=0.1mol��
����̼Ԫ���غ��֪���л�������к���̼ԭ����Ŀ=
=2��
������ԭ����Ŀ=
=4��
0.1mol�л�����Ӻ���Oԭ�����ʵ���=��0.2mol+0.1mol+0.1mol��2-0.1mol��2��=0.3mol���ʷ����к���Hԭ����Ŀ=
=3
�����л���ķ���ʽΪC2H4O3��
�ʴ�Ϊ��C2H4O3��
��3���л���ķ���ʽΪC2H4O3�������ĸ��л���ֱ���������Na��NaOH��Ӧ������Na��NaOH�����ʵ���֮��Ϊ2��1�����л��ﺬ��-COOH��-OH���ҷ�����-OH��-COOH��Ŀ��ȣ����л���Ľṹ��ʽΪ��HO-CH2-COOH��
�ʴ�Ϊ��HO-CH2-COOH���ǻ����Ȼ���
��4��HOCH2COOH ���ǻ����ɱ���������ȩ�ᣬ��Ӧ����ʽΪ��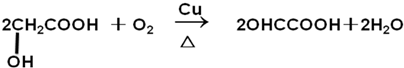 ��
��
�ʴ�Ϊ�� ��
��
��5�����ǻ�����̼�Ϻ�������ɱ�������ȩ���ݴ�д������Ҫ��Ķ����Ľṹ��ʽΪ��CH3CH2CH2CH2OH����CH3��2CH CH2OH��
�ʴ�Ϊ��CH3CH2CH2CH2OH����CH3��2CH CH2OH��
| 3.6g |
| 18g/mol |
���л���ȼ�����ɵ�COΪx����
CuO+CO
| ||
28g 16g
x 1.6g
����x=
| 28g��1.6g |
| 16g |
| 2.8g |
| 28g/mol |
����̼Ԫ���غ��֪CO��CuO��Ӧ���ɵ�CO2�����ʵ���Ϊ0.1mol������Ϊ0.1mol��44g/mol=4.4g��
�л���ȼ�����ɵ�CO2������Ϊ8.8g-4.4g=4.4g�����ʵ���Ϊ
| 4.4g |
| 44g/mol |
�ʴ�Ϊ��0.2��0.1��
��2�����л������Է�������Ϊ��38��2=76��7.6g���л�������ʵ���Ϊ��
| 7.6g |
| 76g/mol |
����̼Ԫ���غ��֪���л�������к���̼ԭ����Ŀ=
| 0.1mol+0.1mol |
| 0.1mol |
������ԭ����Ŀ=
| 0.2mol��2 |
| 0.1mol |
0.1mol�л�����Ӻ���Oԭ�����ʵ���=��0.2mol+0.1mol+0.1mol��2-0.1mol��2��=0.3mol���ʷ����к���Hԭ����Ŀ=
| 0.3mol |
| 0.1mol |
�����л���ķ���ʽΪC2H4O3��
�ʴ�Ϊ��C2H4O3��
��3���л���ķ���ʽΪC2H4O3�������ĸ��л���ֱ���������Na��NaOH��Ӧ������Na��NaOH�����ʵ���֮��Ϊ2��1�����л��ﺬ��-COOH��-OH���ҷ�����-OH��-COOH��Ŀ��ȣ����л���Ľṹ��ʽΪ��HO-CH2-COOH��
�ʴ�Ϊ��HO-CH2-COOH���ǻ����Ȼ���
��4��HOCH2COOH ���ǻ����ɱ���������ȩ�ᣬ��Ӧ����ʽΪ��
 ��
���ʴ�Ϊ��
 ��
����5�����ǻ�����̼�Ϻ�������ɱ�������ȩ���ݴ�д������Ҫ��Ķ����Ľṹ��ʽΪ��CH3CH2CH2CH2OH����CH3��2CH CH2OH��
�ʴ�Ϊ��CH3CH2CH2CH2OH����CH3��2CH CH2OH��
���������⿼���л������ʽ��ȷ���������ŵ����ʵȣ��Ѷ��еȣ�����ԭ���غ��ж��л���ķ���ʽ��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
�����йػ�ѧ���뾧��ṹ��������ȷ���ǣ�������
| A��ˮ�����ɱ��ۻ�ʱ�˷����Ӽ���������������ͬ |
| B��12g���ʯ�У���C-C���ۼ�����Ϊ4mol |
| C�����Ӿ������ۻ�ʱ�����Ӽ����ƻ��������Ӿ����ۻ�ʱ����ѧ�������ƻ� |
| D���۵��ɸߵ��͵�˳���ǣ�����裾̼���裾���ʯ |
NA��ʾ�����ӵ�������ֵ������˵����ȷ���ǣ�������
| A��4.6g���ΪC2H6O���л������C-H����Ŀһ��Ϊ0.6NA |
| B��12g���ʯ�к��еĹ��ۼ���Ϊ2NA |
| C��0.1mol�İ��ף�P4��������������ĦҼ�����Ϊ0.4NA |
D��0.5mol�ۻƣ�As4S4�ṹ��ͼ������NA��S-S��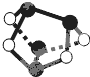 |
�������ʵ�����ṹ��NH3��ͬ���ǣ�������
| A��H2O |
| B��CH4 |
| C��CO2 |
| D��H3O+ |
����˵����ȷ���ǣ�������
A����±����RCH2CH2X�л�ѧ����ͼ ��ʾ��������ȥ��Ӧʱ�����ƻ��ļ��Ǣٺ͢� ��ʾ��������ȥ��Ӧʱ�����ƻ��ļ��Ǣٺ͢� |
| B�������������������������Һ���Ҵ����ͻ������ɲ��÷�Һ���� |
C���л���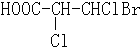 �к���3������̼ԭ�� �к���3������̼ԭ�� |
D�� ��һ������Ҳ�������ʳ�Ϊ��ʽ���飮��ͼ�����ֳ�ʽ���飬���ೲʽ�����ͨʽ��CnHn+4 |
����������ȷ���ǣ�������
| A��ͨ�������ۺ��������Ļ��������ȼ� |
| B��ij������ֻ����һ��Ԫ�أ�������һ���Ǵ����� |
| C��40K��40Ca����������ȡ������������ |
| D����ϩ������̼̼˫���ļ��������������̼̼�������ܵ����� |
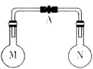 ����ʱ�������ݻ���ͬ����ƿ�зֱ�ʢ��M��N�������壨ͬ��ͬѹ����ȡ�µ��ɼ�A��ʹ����ƿ�ڵ�����Ӵ�����ͼ���������ڵ�ѹǿ�ɴ�С��˳���ǣ�������
����ʱ�������ݻ���ͬ����ƿ�зֱ�ʢ��M��N�������壨ͬ��ͬѹ����ȡ�µ��ɼ�A��ʹ����ƿ�ڵ�����Ӵ�����ͼ���������ڵ�ѹǿ�ɴ�С��˳���ǣ�������| ��� | �� | �� | �� | �� |
| ����M | H2S | H2 | NH3 | NO |
| ����N | SO2 | Cl2 | HCl | O2 |
| A���٢ڢۢ� | B���٢ܢۢ� |
| C���ڢܢ٢� | D���ܢ٢ڢ� |
���з��ӻ������м����ɴ�С����˳���ǣ�������
��BF3 ��NH3 ��H2O ��NH4+ ��BeCl2��
��BF3 ��NH3 ��H2O ��NH4+ ��BeCl2��
| A���ݢܢ٢� | B���ݢ٢ܢڢ� |
| C���ܢ٢ڢݢ� | D���ۢڢܢ٢� |