��Ŀ����
2����������14��Ԫ�صĵ縺�ԣ�| Ԫ�� | Al | B | Be | C | Cl | F | Li |
| �縺�� | 1.5 | 2.0 | 1.5 | 2.5 | 3.0 | 4.0 | 1.0 |
| Ԫ�� | Mg | N | Na | O | P | S | Si |
| �縺�� | 1.2 | 3.0 | 0.9 | 3.5 | 2.1 | 2.5 | 1.8 |
��1�����ݱ��и��������ݣ�����֪Ԫ�صĵ縺�Ծ��еı仯��������ͬһ�����У�����ԭ�������ĵ�����Ԫ�صĵ縺���������������Ա仯����
��2���ж��������������ӻ����ﻹ�ǹ��ۻ����
Mg3N2�����ӻ����BeCl2�����ۻ����AlCl3�����ۻ����SiC�����ۻ����
���� ��1����ͬ����Ԫ�����������У�Ȼ�������ݷ�����
��2�������γɻ�ѧ������ԭ����ӦԪ�صĵ縺�Բ�ֵ����1.7ʱ�����γɵ�һ��Ϊ���Ӽ�����С��1.7ʱ��һ��Ϊ���ۼ�������
��� �⣺��1���ɱ������ݿ�֪���ڶ�����Ԫ�ش�Li��F������ԭ�������ĵ�����Ԫ�صĵ縺��������������Ԫ�ش�Na��S������ԭ�������ĵ�����Ԫ�صĵ縺��Ҳ�������������Ա仯��
�ʴ�Ϊ����ͬһ�����У�����ԭ�������ĵ�����Ԫ�صĵ縺���������������Ա仯��
��2��Ԫ�صĵ縺����Ԫ�صĻ������ʣ�������ԭ�������ĵ����������Ա仯��
Mg3N2�縺�Բ�ֵΪ3.0-1.2=1.8������1.7�γ����Ӽ����������ӻ����
BeCl2�縺�Բ�ֵΪ3.0-1.5=1.5��С����1.7�γɹ��ۼ������ڹ��ۻ����
AlCl3�縺�Բ�ֵΪ3.0-1.5=1.5��С����1.7�γɹ��ۼ������ڹ��ۻ����
SiC�縺�Բ�ֵΪ2.5-1.8=0.7��С����1.7�γɹ��ۼ������ڹ��ۻ����
�ʴ�Ϊ�����ӻ�������ۻ�������ۻ�������ۻ����
���� ���⿼���˵縺�Եĺ����Ӧ�ã���Ŀ�ѶȲ���ע��һ������µ縺�Եı仯������Ԫ�طǽ����Եı仯����һ�£�

��ϰ��ϵ�д�
 Сѧ��ʱ��ѵϵ�д�
Сѧ��ʱ��ѵϵ�д�
�����Ŀ
9��������������ȷ���ǣ�������
| A�� | ����������ˮ������ˮ�м�����������ʹ��ˮ���� | |
| B�� | ���ӽ���Ĥ�ڹ�ҵ��Ӧ�ù㷺�����ȼҵ��ʹ�������ӽ���Ĥ | |
| C�� | ������һ�������Խ�ǿ�����壬����������ˮ������ | |
| D�� | SO2���л�ԭ�ԣ�������֪Ũ�ȵ�KMnO4��Һ�ⶨʳƷ��SO2������ |
7������˵�����ʾ������ȷ���ǣ�������
| A�� | �����ʵ������������������ֱ���ȫȼ�գ����߷ų������� | |
| B�� | ��H+��aq��+OH-��aq��=H2O��l����H=-57.3kJ•mol-1��֪��������1 mol CH3COOH��ϡ��Һ�뺬1 mol NaOH��ϡ��Һ��ϣ��ų�������С��57.3kJ | |
| C�� | 300�桢30MPa�£���0.5molN2��g����1.5mol H2��g�������ܱ������г�ַ�Ӧ����NH3��g��������19.3kJ�����Ȼ�ѧ����ʽΪ��N2��g��+3H2��g��?2NH3��g����H=-38.6kJ•mol-1 | |
| D�� | ��C��ʯī��=C�����ʯ����H=+1.90 kJ•mol-1��֪�����ʯ��ʯī�ȶ� |
14��C60��60��̼ԭ���γɵķ����״���ӣ��������� ������ʯī��Ϊ��������
������ʯī��Ϊ��������
 ������ʯī��Ϊ��������
������ʯī��Ϊ��������| A�� | ͬλ�� | B�� | ͬ�������� | C�� | ͬ�ַ��� | D�� | ͬһ���� |
11�����������������ֵ���ֱ�ӻ��ϵõ����ǣ�������
| A�� | FeCl2 | B�� | CuS | C�� | NO2 | D�� | SO2 |
12�����и����е�X��Y����ԭ�ӣ���ѧ����һ�����Ƶ��ǣ�������
| A�� | Xԭ�Ӻ�Yԭ������㶼ֻ��һ������ | |
| B�� | Xԭ��2p�ܼ������������ӣ�Yԭ�ӵ�3p�ܼ������������� | |
| C�� | Xԭ�ӵĺ�������Ų�Ϊ1s2��Yԭ�ӵĺ�������Ų�Ϊ1s22s2 | |
| D�� | Xԭ�Ӻ���M���Ͻ����������ӣ�Yԭ�Ӻ���N���Ͻ����������� |
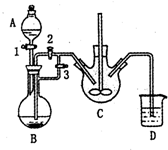 ����������[CH3CH��OH��COO]2Fe•3H2O��Mr=288����һ�ֳ��õIJ���������ͨ��������̼��������Ӧ�Ƶã�
����������[CH3CH��OH��COO]2Fe•3H2O��Mr=288����һ�ֳ��õIJ���������ͨ��������̼��������Ӧ�Ƶã�