��Ŀ����
ij��Һ��ֻ���ܺ������������еļ��֣�K+��NO3-��SO42-��NH4+��CO32-����������Һ��������H+��OH-����ȡ200mL����Һ����Ϊ���ȷݽ�������ʵ�飺����˵����ȷ���ǣ�������
ʵ��1����һ�ݼ����������ռ���ȣ������������ڱ�״����Ϊ224mL��
ʵ��2���ڶ����ȼ������������ᣬ�������ټ���������BaCl2��Һ���ù���2.33g��
ʵ��1����һ�ݼ����������ռ���ȣ������������ڱ�״����Ϊ224mL��
ʵ��2���ڶ����ȼ������������ᣬ�������ټ���������BaCl2��Һ���ù���2.33g��
| A������Һ�п��ܺ�K+ |
| B������Һ�п϶�����NO3?��SO42-��NH4+��CO32- |
| C������Һ��һ������NH4+ |
| D������Һ��һ����K+����c��K+����0.1mol/L |
���㣺���ʵļ���ͼ���Ļ�������ѡ��Ӧ��,���������ӵļ���,���������ӵļ���
ר�⣺���ʵ���Ũ�Ⱥ��ܽ��ר��
��������ʵ��1��֪����һ�ݼ����������ռ���Ȼ�����ڱ�״����Ϊ224mL����֤������NH4+�������ʵ���Ϊ0.01mol��
��ʵ��2��֪���ڶ����ȼ������������ᣬ����������CO32-���ټ�������BaCl2��Һ���ù���2.33g��֤������SO42-�������ʵ���Ϊ0.01mol��������Һ�ʵ�����ԭ�����ж��Ƿ���NO3-���Դ������
��ʵ��2��֪���ڶ����ȼ������������ᣬ����������CO32-���ټ�������BaCl2��Һ���ù���2.33g��֤������SO42-�������ʵ���Ϊ0.01mol��������Һ�ʵ�����ԭ�����ж��Ƿ���NO3-���Դ������
���
�⣺��ʵ��1��֪����һ�ݼ����������ռ���Ȼ�����ڱ�״����Ϊ224mL����֤������NH4+�������ʵ���Ϊ
=0.01mol��
��ʵ��2��֪���ڶ����ȼ������������ᣬ����������CO32-���ټ�������BaCl2��Һ���ù���2.33g��֤������SO42-�������ʵ���Ϊ
=0.01mol��
������Һ�еĵ���غ㣬��Һ�ʵ����ԣ���һ�����м����ӣ����Ը���Һ�п϶�����NH4+��S042-��K+��
���K+�����ʵ�������0.01mol����NO3-�����K+�����ʵ�������0.01mol����Ӧ����NO3-��
A��������������֪һ�����м����ӣ���A����
B���ɷ�����֪̼�������һ�������ڣ���������ӿ��ܴ��ڣ���B����
C����Һ��һ����NH4+����Ϊ0.01mol����C����
D���ɵ���غ��֪��һ������K+��c��K+����
=0.1 mol/L����D��ȷ��
��ѡD��
| 0.224L |
| 22.4L/mol |
��ʵ��2��֪���ڶ����ȼ������������ᣬ����������CO32-���ټ�������BaCl2��Һ���ù���2.33g��֤������SO42-�������ʵ���Ϊ
| 2.33g |
| 233g/mol |
������Һ�еĵ���غ㣬��Һ�ʵ����ԣ���һ�����м����ӣ����Ը���Һ�п϶�����NH4+��S042-��K+��
���K+�����ʵ�������0.01mol����NO3-�����K+�����ʵ�������0.01mol����Ӧ����NO3-��
A��������������֪һ�����м����ӣ���A����
B���ɷ�����֪̼�������һ�������ڣ���������ӿ��ܴ��ڣ���B����
C����Һ��һ����NH4+����Ϊ0.01mol����C����
D���ɵ���غ��֪��һ������K+��c��K+����
| 0.01mol��2-0.01mol |
| 0.1L |
��ѡD��
���������⿼�����ʵļ���ʵ�������Ϊ��Ƶ���㣬���ճ�������֮��ķ�Ӧ��������������Ӽ��顢����غ��Ϊ���Ĺؼ������ط�����ʵ�������Ŀ��飬��Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
ҽ������ij��״���״�IJ��˶�ʳ�ú������������ں����к��нϷḻ�ģ�������
| A����Ԫ�� | B����Ԫ�� |
| C��ά���� | D����Ԫ�� |
�����йص�������ȷ���ǣ�������
A�� 2��3���� |
B�� 3�һ�1��ϩ |
| C����CH3CH2��2CHCH3��3������ |
D�� ������ |





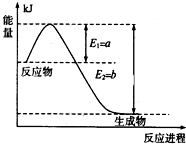 ���ǵ����Ϻ����ḻ��һ��Ԫ�أ������仯�����ڹ�ũҵ������������������Ҫ���ã�
���ǵ����Ϻ����ḻ��һ��Ԫ�أ������仯�����ڹ�ũҵ������������������Ҫ���ã�