��Ŀ����
7����ԭ�������ĵ��������ֶ�����Ԫ�أ�����ĸ��ʾ��ԭ�Ӱ뾶����Դ�С��������ۻ�����۵ı仯��ͼ1��ʾ��
�����жϳ���Ԫ�ػش����⣺
��1��f��Ԫ�����ڱ���λ���ǵ������ڢ�A�壮
��2���Ƚ�d��e�������ӵİ뾶�Ĵ�С���û�ѧʽ��ʾ����ͬ��O2-��Na+���Ƚ�g��h������������Ӧ��ˮ���������ǿ���ǣ�HClO4��H2SO4��
��3��������ӣ�yz��2�ĵ���ʽΪ
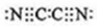 ����yz��2��Ϊ��±�أ�������±�����ƣ���������������Һ��Ӧ�Ļ�ѧ����ʽ��2NaOH+��CN��2=NaCN+NaCNO+H2O��
����yz��2��Ϊ��±�أ�������±�����ƣ���������������Һ��Ӧ�Ļ�ѧ����ʽ��2NaOH+��CN��2=NaCN+NaCNO+H2O����4��hd2�dz�����ˮ�ľ�������ҵ�Ͽ���h2����ehd2��Һ��ȡhd2��д���÷�Ӧ�����ӷ���ʽ�����������ת�Ƶķ������Ŀ
 ��
����5������Ԫ�ؿ������R��zx4f��gd4��2����ʢ��10mL1mol•L-1R��Һ���ձ��еμ�1mol•L-1NaOH��Һ���������ʵ�����NaOH��Һ����仯ʾ��ͼ2���£�
��д��m�㷴Ӧ�����ӷ���ʽNH4++OH-=NH3•H2O��
����R��Һ�ļ�20mL1.2mol•L-1Ba��OH��2��Һ����ַ�Ӧ����Һ�в������������ʵ���Ϊ0.022mol��
���� ��ͼ�еĻ��ϼۡ�ԭ�Ӱ뾶�Ĵ�С��ԭ����������֪x��HԪ�أ�y��CԪ�أ�z��NԪ�أ�d��OԪ�أ�e��NaԪ�أ�f��AlԪ�أ�g��SԪ�أ�h��ClԪ�أ�
��1��f��AlԪ�أ���Ԫ�����ڱ���λ���ǵ������ڢ�A�壻
��2�����Ӳ�ṹ��ͬ�����ӣ��˵����Խ�����Ӱ뾶ԽС���ǽ�����Խǿ�����������ˮ���������Խǿ��
��3����ԭ�ӹ��ۻ����������NH3��H2O2��C2H2�ȣ�
��4��Cl2����NaClO2��Һ��ȡClO2����������ԭΪ�����ӣ�1���������ӷ�Ӧ�õ�2�����ӣ�
��5����R��NH4Al��SO4��2��m������м����������ƣ��������ʵ������䣬��NH4+��OH-��Ӧ����NH3•H2O��
�ڸ���n=cV����n��Al3+ ����n��NH4+����n��SO42-����n��Ba2+����n��OH-��������SO42-��Ba2+�в����������ӵ����ʵ�����������BaSO4�����ʵ��������η�����Al3++3OH-=Al��OH��3����NH4++OH-=NH3•H2O��Al��OH��3+OH-=AlO2-+2H2O�����ݷ���ʽ��������Al��OH��3�����ʵ������������������ɹ��������ʵ�����
��� �⣺��ͼ�еĻ��ϼۡ�ԭ�Ӱ뾶�Ĵ�С��ԭ����������֪x��HԪ�أ�y��CԪ�أ�z��NԪ�أ�d��OԪ�أ�e��NaԪ�أ�f��AlԪ�أ�g��SԪ�أ�h��ClԪ�أ�
��1��f��AlԪ�أ���Ԫ�����ڱ���λ���ǵ������ڢ�A�壬
�ʴ�Ϊ���������ڢ�A�壻
��2�����Ӳ�ṹ��ͬ�����ӣ��˵����Խ�����Ӱ뾶ԽС�������Ӱ뾶��O2-��Na+���ǽ�����Խǿ�����������ˮ���������Խǿ�������ԣ�HClO4��H2SO4��
�ʴ�Ϊ��O2-��Na+��HClO4��H2SO4��
��3������ӣ�yz��2Ϊ��CN��2������ʽΪ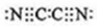 ����������������������Һ��Ӧ֪����CN��2��NaOH��Ӧ����NaCN��NaCNO��H2O����Ӧ����ʽΪ2NaOH+��CN��2=NaCN+NaCNO+H2O��
����������������������Һ��Ӧ֪����CN��2��NaOH��Ӧ����NaCN��NaCNO��H2O����Ӧ����ʽΪ2NaOH+��CN��2=NaCN+NaCNO+H2O��
�ʴ�Ϊ��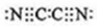 ��2NaOH+��CN��2=NaCN+NaCNO+H2O��
��2NaOH+��CN��2=NaCN+NaCNO+H2O��
��4��Cl2����NaClO2��Һ��ȡClO2����������ԭΪ�����ӣ�1���������ӷ�Ӧ�õ�2�����ӣ�������ӷ���ʽ������ת�Ƶķ������Ŀ��ʾΪ�� ��
��
�ʴ�Ϊ�� ����
����
��5����R��NH4Al��SO4��2��m������м����������ƣ��������ʵ������䣬��NH4+��OH-��Ӧ����NH3•H2O�����ӷ���ʽΪ��NH4++OH-=NH3•H2O��
�ʴ�Ϊ��NH4++OH-=NH3•H2O��
��10mL 1mol•L-1 NH4Al��SO4��2��Һ��Al3+ ���ʵ���Ϊ0.01mol��NH4+�����ʵ���Ϊ0.01mol��SO42-�����ʵ���Ϊ0.02mol��20mL 1.2 mol•L-1Ba��OH��2��Һ��Ba2+���ʵ���Ϊ0.024mol��OH-Ϊ0.048mol��
��SO42-+Ba2+=BaSO4������֪SO42-���㣬�ʿ��Եõ�0.02mol BaSO4��
Al3++3OH-=Al��OH��3��
0.01mol 0.03mol 0.01mol
��Ӧʣ��OH-Ϊ0.048mol-0.03mol=0.018mol��
NH4++OH-=NH3•H2O
0.01mol 0.01mol
��Ӧʣ��OH-Ϊ0.018mol-0.01mol=0.008mol��
Al��OH��3+OH-=AlO2-+2H2O
0.008mol 0.008mol
�ʵõ�Al��OH��3����Ϊ0.01mol-0.008mol=0.002mol
�����յõ�����Ϊ0.02mol+0.002mol=0.022mol��
�ʴ�Ϊ��0.022��
���� ���⿼��λ�á��ṹ�����ʵĹ�ϵ����Ŀ�Ѷ��еȣ��漰ԭ�ӽṹ��Ԫ�������ɡ���Ӧ�ȵļ��㡢���ӷ���ʽ��д��������ԭ��ת�Ƶ������ļ���ͱ�ʾ������Ϊ�߿��������ͣ�������ѧ�����������������Ƚ�������������

 ��У����ϵ�д�
��У����ϵ�д� ������λͬѧһ����ˮ����ص�ʵ�飬����������£�
������λͬѧһ����ˮ����ص�ʵ�飬����������£�| ������ | �缫���� | ˮ��Ʒ�� | �缫���/cm | ��ѹ/mV | |
| 1 | п | ͭ | ���� | 3 | 900 |
| 2 | п | ͭ | ƻ�� | 3 | 650 |
| 3 | п | ͭ | �̽� | 3 | 850 |
| 4 | п | ͭ | ������ | 3 | 750 |
| 5 | п | �� | ���� | 3 | 650 |
| 6 | п | �� | ƻ�� | 3 | 450 |
�ټף�ʵ��6�и����缫��Ӧʽ���д��
�ң���Ϊ������Al-3e-=Al3+
�ڼף�ʵ��1��5�е�������Ϊʲô�෴��
�ң�ʵ��1��п��ͭ���ã�пΪ������ʵ��5����Ϊ��������ʧȥ���ӣ�������п������������
�ۼף�ˮ����صĵ�ѹ����Щ�����йأ�
�ң�ֻ��ˮ����Ʒ���йأ�
| A�� | �� | B�� | �� | C�� | �٢ڢ� | D�� | �ڢ� |
| A�� | pH��7����Һ�������Һ | |
| B�� | pH��7����Һ�Ǽ����Һ | |
| C�� | pH��7����Һ�п������ε���Һ��������Ϊ�ο��ܵ����H+ | |
| D�� | pH��7����Һ�п������ε���Һ����������ˮ����ɵ� |
�й����������ʾ��
| �¶�/�� ������ | 0 | 20 | 40 | 60 | 80 | 100 |
| NH4Cl | 29.3 | 37.2 | 45.8 | 55.3 | 65.6 | 77.3 |
| ZnCl2 | 343 | 395 | 452 | 488 | 541 | 614 |

| A�� | �õ�ص�������ӦʽΪMnO2+e��+H+�TMnOOH | |
| B�� | ���øɵ�أ����µ��H2O-CO2��������Ʊ�H2��CO����ͼb�����������������ɵ���������ʵ���֮����1��1 | |
| C�� | �ϵ�غ�״������ˮ�������ˣ���Һ����Ҫ���Ȼ�п���Ȼ�泥����߿���ͨ���ؽᾧ�������� | |
| D�� | �ϵ�غ�״������ˮ������������������Ҫ�ɷ��Ƕ������̡�̼�ۺ�MnOOH�������еõ��ϴ��Ķ������̣����Բ��ü��ȵķ��� |
| A�� | ����ʯ��ˮ��̼����Ʒ�Ӧ��Ca2++2HCO3-+2OH-�T=CaCO3��+CO32-+2H2O | |
| B�� | FeSO4������Һ��¶�ڿ����У�4Fe2++O2+4H+�T4Fe3++2H2O | |
| C�� | ��ϡHNO3�еμ�Na2SO3��Һ��SO32-+2H+�TSO2��+H2O | |
| D�� | ������Һ��KlO3��KI��Ӧ����I2��IO3-+I-+6H+�TI2+3H2O |
| A�� | ��KSCN��Һ����FeCl2��FeCl3��Һ | |
| B�� | �ó���ʯ��ˮ����SO2��CO2 | |
| C�� | ����ɫ��Ӧ����NaCl��Һ��KCl��Һ | |
| D�� | �ù�����NaOH��Һ����AlCl3��Һ��MgCl2��Һ |

| A�� | װ�â��У����ţ�����������KCl������Һ���е�K+����ZnSO4��Һ | |
| B�� | װ�âڹ���һ��ʱ���a��������Һ��pH��С | |
| C�� | ��װ�â۾���ͭʱ��c��Ϊ��ͭ | |
| D�� | װ�â��е�����Zn����Fe��װ������Fe2+���� |

 ��������������أ����������ڣ��ؿ��д�Լ5%�������Ǻ������������Ľ��������ĵ��ʼ��������ڿ�ѧ�о���ҵ�����о���������;��������ѧ֪ʶ�ش��������⣺
��������������أ����������ڣ��ؿ��д�Լ5%�������Ǻ������������Ľ��������ĵ��ʼ��������ڿ�ѧ�о���ҵ�����о���������;��������ѧ֪ʶ�ش��������⣺