��Ŀ����
3�� ��͵�Ԫ���ڻ�ѧ���к���Ҫ�ĵ�λ���ش��������⣺
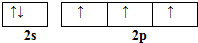
��͵�Ԫ���ڻ�ѧ���к���Ҫ�ĵ�λ���ش��������⣺��1����̬��ԭ�Ӻ��������5�ֲ�ͬ���˶�״̬����̬��ԭ�ӵļ۲�����Ų�ͼΪ
 ��Ԥ����2017�귢��ġ��϶���š�̽�������õij���5�����ػ��ȼ��Ϊƫ������[��CH3��2NNH2]����CH3��2NNH2��Nԭ�ӵ��ӻ���ʽΪsp3��
��Ԥ����2017�귢��ġ��϶���š�̽�������õij���5�����ػ��ȼ��Ϊƫ������[��CH3��2NNH2]����CH3��2NNH2��Nԭ�ӵ��ӻ���ʽΪsp3����2��������H3BNH3��һ��DZ�ڵĴ�����ϣ������û�����B3N3H6ͨ�����·�Ӧ�Ƶã�3CH4+2B3N3H6+6H2O=3CO2+6H3BNH3
��H3BNH3�������Ƿ������λ���ǣ���ǡ�����B��C��N��O�ĵ�һ��������С�����˳��ΪB��C��O��N��
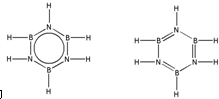
����B3N3H6��Ϊ�ȵ�����ķ�����C6H6����һ�����ɣ���B3N3H6Ϊ�Ǽ��Է��ӣ����ݵȵ���ԭ��д��B3N3H6�Ľṹʽ
 ����
������3�����϶���š�̽��������̫���ܵ�ذ��ṩ��������̫���ܵ�ذ�����г��������⣬����ͭ�������أ����Ȼ�ѧ���ʣ��ش��������⣺
��SeO3���ӵ����幹��Ϊƽ���������Σ�
�ڽ���ͭͶ�백ˮ��H2O2��Һ�о�����������Ͷ�백ˮ��H2O2�Ļ����Һ�У���ͭƬ�ܽ⣬��Һ������ɫ��д���÷�Ӧ�����ӷ�Ӧ����ʽΪCu+H2O2+4NH3•H2O=Cu��NH3��42++2OH-+4H2O��
��ij��ͭ�Ͻ�ľ����ṹ��ͼ��ʾ���þ����о��������ͭԭ�Ӻ͵�ԭ�Ӽ�ľ���Ϊ$\frac{\sqrt{2}}{2}$a pm����þ�����ܶ�Ϊ$\frac{206}{{N}_{A}����\sqrt{2}a��1{0}^{-10}��^{3}}$g•cm-3���ú�a�Ĵ���ʽ��ʾ����NAΪ����٤��������ֵ����
���� ��1������û���˶�״̬��ͬ�ĵ��ӣ�Nԭ�Ӽ۵����Ų�ʽΪ2s22p3���������ԭ�������ع����۵����Ų�ͼ����CH3��2NNH2��2��Nԭ�Ӿ��γ�3������������1�Թ¶Ե��ӣ��۲���Ӷ�����Ϊ4��
��2����Bԭ�Ӽ۵�����Ϊ3��B�γ�3������Nԭ�Ӽ۵�����Ϊ5��Ҳ�γ�3��������H3BNH3������B��N���γ�4������Bԭ���пչ����Nԭ�Ӻ��й¶Ե��ӣ��γ�1����λ����
ͬһ����Ԫ�صĵ�һ����������ԭ����������������������ƣ�����IIA�塢��VA��Ԫ�صĵ�һ�����ܴ�������Ԫ�صģ�
��ԭ������ͬ���۵���������ͬ����Ϊ�ȵ����壬B3N3H6��C6H6��Ϊ�ȵ����壬���߽ṹ���������ƣ�
��3����SeO3������Seԭ�ӹµ��Ӷ���=$\frac{6-2��3}{2}$=0���۲���Ӷ���Ϊ3+0=3��
��CuͶ�백ˮ��H2O2�Ļ����Һ�У���ͭƬ�ܽ⣬��Һ������ɫ������[Cu��NH3��4]2+���ɵ���غ��֪������OH-����ƽ��д���ӷ���ʽ��
�۸þ����о��������ͭԭ�Ӻ͵�ԭ�Ӽ�ľ���Ϊ$\frac{\sqrt{2}}{2}$a pm�����ⳤΪ$\sqrt{2}$a pm�����ݾ�̯�����㾧����Cu��Nԭ����Ŀ����ʾ���������������ٸ��ݦ�=$\frac{m}{V}$���㾧���ܶȣ�
��� �⣺��1��Bԭ�Ӻ��������Ϊ5������û���˶�״̬��ͬ�ĵ��ӣ���̬��ԭ�Ӻ��������5�ֲ�ͬ���˶�״̬��
Nԭ�Ӽ۵����Ų�ʽΪ2s22p3���������ԭ�������ع���֪�۵����Ų�ͼΪ ��
��
��CH3��2NNH2��2��Nԭ�Ӿ��γ�3������������1�Թ¶Ե��ӣ��۲���Ӷ�����Ϊ4������N���ӻ���ʽΪsp3�ӻ���
�ʴ�Ϊ��5�� ��sp3��
��sp3��
��2����Bԭ�Ӽ۵�����Ϊ3��B�γ�3������Nԭ�Ӽ۵�����Ϊ5��Ҳ�γ�3��������H3BNH3������B��N���γ�4������Bԭ���пչ����Nԭ�Ӻ��й¶Ե��ӣ��γ�1����λ����
ͬһ����Ԫ�صĵ�һ����������ԭ����������������������ƣ�����IIA�塢��VA��Ԫ�صĵ�һ�����ܴ�������Ԫ�صģ��������ǵĵ�һ������˳����B��C��O��N��
�ʴ�Ϊ���ǣ�B��C��O��N��
��ԭ������ͬ���۵���������ͬ����Ϊ�ȵ����壬B3N3H6��C6H6��Ϊ�ȵ����壬���߽ṹ���������ƣ�B3N3H6�ĽṹʽΪ�� ��
��
�ʴ�Ϊ��C6H6�� ��
��
��3����SeO3������Seԭ�ӹµ��Ӷ���=$\frac{6-2��3}{2}$=0���۲���Ӷ���Ϊ3+0=3���ռ�ṹΪƽ���������Σ�
�ʴ�Ϊ��ƽ���������Σ�
��CuͶ�백ˮ��H2O2�Ļ����Һ�У���ͭƬ�ܽ⣬��Һ������ɫ������[Cu��NH3��4]2+���ɵ���غ��֪������OH-����Ӧ���ӷ���ʽΪ��Cu+H2O2+4NH3•H2O=Cu��NH3��42++2OH-+4H2O��
�ʴ�Ϊ��Cu+H2O2+4NH3•H2O=Cu��NH3��42++2OH-+4H2O��
�۸þ����о��������ͭԭ�Ӻ͵�ԭ�Ӽ�ľ���Ϊ$\frac{\sqrt{2}}{2}$a pm�����ⳤΪ$\sqrt{2}$a pm���������V=��$\sqrt{2}$a��10-10cm��3���ھ����У�Nԭ��λ�ڶ��㣬Cuԭ��λ�����е㣬�þ�����Nԭ�Ӹ���=8��$\frac{1}{8}$=1��Cuԭ�Ӹ���=12��$\frac{1}{4}$=3��������Ϊ$\frac{14+64��3}{{N}_{A}}$g���ʾ����ܶ�Ϊ$\frac{14+64��3}{{N}_{A}}$g�£�$\sqrt{2}$a��10-10cm��3=$\frac{206}{{N}_{A}����\sqrt{2}a��1{0}^{-10}��^{3}}$g•cm-3��
�ʴ�Ϊ��$\frac{206}{{N}_{A}����\sqrt{2}a��1{0}^{-10}��^{3}}$g•cm-3��
���� �����Ƕ����ʽṹ�����ʵĿ��飬�漰��������Ų����ӻ���ʽ��ռ乹���жϡ������ܡ���λ�����ȵ����塢����ṹ�����ȣ���Ŀ�ۺ���ǿ������������ѧ����֪ʶ��Ǩ������������


����˵������ȷ���ǣ�������
| A�� | �ô˷���ȡþ���ŵ�֮һ��ԭ����Դ�ḻ | |
| B�� | ���MgCl2ʱ��������þ | |
| C�� | ����ݿɽ���������HCl�����Χ����ˮ����ˮ�Ȼ�þ | |
| D�� | ���������������漰�����ϡ��ֽ���ֽⷴӦ |
| A�� | X��W��U������������Ӧ��ˮ����������ǿ������˳��Ϊ��U��W��X | |
| B�� | Y��ZԪ�صĵ������缫��������������Һ�й���ԭ��أ�Z�缫�ϲ����������� | |
| C�� | �����£�0.05��mol•L-1U����̬�⻯���ˮ��Һ��pH��1 | |
| D�� | Y��Z��UԪ�صļ����Ӱ뾶�ɴ�С��˳��Y��Z��U |
| A�� | Y���⻯��ķе��R���⻯��ķе�� | |
| B�� | Z��W��R������������Ӧˮ���������ǿ������˳����R��W��Z | |
| C�� | X2Y2�������еĻ�ѧ����X2R�еĻ�ѧ��������ȫ��ͬ | |
| D�� | RY2ͨ��Ba��NO3��2��Һ���а�ɫ�������ɣ��ó������������� |
 ��������һ������������ֲ�D�أ����ܴ�ʹҶ���������������ṹ��ͼ��ʾ�����й��������˵����ȷ���� ��������
��������һ������������ֲ�D�أ����ܴ�ʹҶ���������������ṹ��ͼ��ʾ�����й��������˵����ȷ���� ��������| A�� | ������Ļ�ѧʽC15H18O4 | |
| B�� | ������ֻ�ܺʹ����������Ӧ | |
| C�� | 1 mol �����������Ժ�2 mol �����Ʒ�����Ӧ | |
| D�� | 1 mol �����������Ժ�2 mol ���������ӳɷ�Ӧ |


 ��
�� ��д���ϳ�����ͼ�����Լ����ã����ϳ�����ͼʾ�����£�CH2=CH2$\stackrel{HBr}{��}$CH3CH2Br$��_{��}^{NaOH��Һ}$CH3CH2OH��
��д���ϳ�����ͼ�����Լ����ã����ϳ�����ͼʾ�����£�CH2=CH2$\stackrel{HBr}{��}$CH3CH2Br$��_{��}^{NaOH��Һ}$CH3CH2OH��
