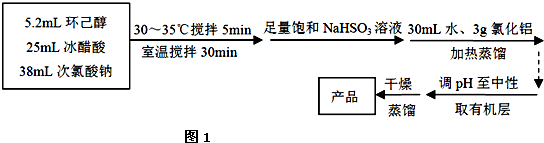
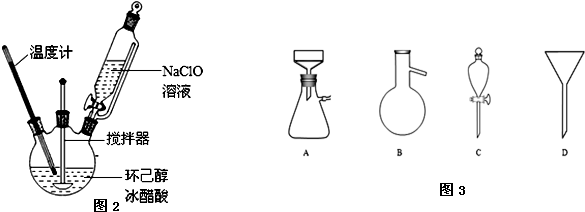
��Ŀ����
W��X��Y��ZΪ�������ڳ�ϡ���������4��Ԫ�أ����ǵ�ԭ������������������ֻ��YΪ����Ԫ�أ�Y��W��������������ȣ�Y��Z��Ԫ��ԭ�ӵ�������֮��ΪW��X��Ԫ��������֮�͵�3�����ɴ˿�֪��
��1��д��Ԫ�����ƣ�W ��X ��Y ��Z ��Z��Ԫ�����ڱ��е�λ�� ��
��2��W2X2�Ľṹʽ ��
��3�����־�������Ԫ�صĻ��������Ӧ�ų�����Ļ�ѧ����ʽ�� ��
��1��д��Ԫ�����ƣ�W
��2��W2X2�Ľṹʽ
��3�����־�������Ԫ�صĻ��������Ӧ�ų�����Ļ�ѧ����ʽ��
���㣺λ�ýṹ���ʵ����ϵӦ��
ר�⣺Ԫ����������Ԫ�����ڱ�ר��
�����������ڵĽ���ֻ��Li��Be��Na��Mg��Al��Y��W��������������ȼ�W��Yͬ�壬����Wֻ����H���⣩������ϡ�����壬�������Y��Li��X�ͳ���ϡ�����壬������������Y��Na��Y����������11��Zһ������Y��Ҳ����12���ϣ���Y��Z�ĺ��ܱ�3���������У�24��27����Z������Ϊ��24-11=13����27-11=16��W����������1��X���������п�����7��8����W��Z��Y��Z�ֱ�Ϊ��1��7��11��13����Ϊ��һ�ֽ���������������1��8��11��16 ����������W��X��Y��Z�ֱ�Ϊ��H��O��Na��S���ݴ˻ش��С�⼴�ɣ�
���
�⣺�����ڵĽ���ֻ��Li��Be��Na��Mg��Al��Y��W��������������ȼ�W��Yͬ�壬����Wֻ����H���⣩������ϡ�����壬�������Y��Li��X�ͳ���ϡ�����壬������������Y��Na��Y����������11��Zһ������Y��Ҳ����12���ϣ���Y��Z�ĺ��ܱ�3���������У�24��27����Z������Ϊ��24-11=13����27-11=16��W����������1��X���������п�����7��8����W��Z��Y��Z�ֱ�Ϊ��1��7��11��13����Ϊ��һ�ֽ���������������1��8��11��16 ����������W��X��Y��Z�ֱ�Ϊ��H��O��Na��S��
����������WΪ�⣬XΪ����YΪ�ƣ�ZΪ��
��1��H��O��Na��S�����Ʒֱ�Ϊ���⣬�����ƣ���ZΪ��S���ڵ������ڵڢ�A�壬�ʴ�Ϊ���⣻�����ƣ��������ڵڢ�A�壻
��2��W2X2ΪH2O2��˫��ˮ��H-O�γɹ��ۼ���O-O�γɷǼ��Թ��ۼ�������ṹʽΪ��H-O-O-H���ʴ�Ϊ��H-O-O-H��
��3�����־���H��O��Na��S����Ԫ�صĻ�����ֱ�Ϊ���������ƺ����������ƣ��������Ӧ�ų�����Ļ�ѧ����ʽ�ǣ�NaHSO3+NaHSO4=Na2SO4+SO2��+H2O��
�ʴ�Ϊ��NaHSO3+NaHSO4=Na2SO4+SO2��+H2O��
����������WΪ�⣬XΪ����YΪ�ƣ�ZΪ��
��1��H��O��Na��S�����Ʒֱ�Ϊ���⣬�����ƣ���ZΪ��S���ڵ������ڵڢ�A�壬�ʴ�Ϊ���⣻�����ƣ��������ڵڢ�A�壻
��2��W2X2ΪH2O2��˫��ˮ��H-O�γɹ��ۼ���O-O�γɷǼ��Թ��ۼ�������ṹʽΪ��H-O-O-H���ʴ�Ϊ��H-O-O-H��
��3�����־���H��O��Na��S����Ԫ�صĻ�����ֱ�Ϊ���������ƺ����������ƣ��������Ӧ�ų�����Ļ�ѧ����ʽ�ǣ�NaHSO3+NaHSO4=Na2SO4+SO2��+H2O��
�ʴ�Ϊ��NaHSO3+NaHSO4=Na2SO4+SO2��+H2O��
���������⿼��ԭ�ӽṹ��Ԫ�������ɵĹ�ϵ��Ԫ�ص��ƶϣ���Ŀ�Ѷ��еȣ���ȷ�ƶ�Ԫ�ص�����Ϊ������Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ
 X��Y��Z��W��Ϊ���������ĺ������������������ͬ������֮�������ͼ��ʾ��ת����ϵ�������ж���ȷ���ǣ�������
X��Y��Z��W��Ϊ���������ĺ������������������ͬ������֮�������ͼ��ʾ��ת����ϵ�������ж���ȷ���ǣ�������| A��X�����ᣬY���� |
| B��Z��ȩ��W������ |
| C��Y��ȩ��W�Ǵ� |
| D��X�Ǵ���Z���� |
���������Ƶı���Һ�ζ��������Ũ��ʱ���÷�̪��ָʾ�����ﵽ�ζ��յ�ʱ����Һ����ɫ�仯�ǣ�������
| A������ɫ��Ϊ��ɫ |
| B������ɫ��Ϊdz��ɫ |
| C���ɺ�ɫ��Ϊ��ɫ |
| D���ɺ�ɫ��Ϊdz��ɫ |