��Ŀ����
10����Ҫ��ش����⣮��1����ϩ�ĵ���ʽΪ
 ��
����2����0.2mol��A��������������ȫȼ��ʱ����CO2��H2O��1.2mol�������������
2��2-�������飬��A�Ľṹ��ʽΪ��CH3��3C-CH=CH2��
��3��ij��1mol��2molHCl��ȫ�ӳɣ����ɵ��ȴ�����������4molCl2��Ӧ��������Ľṹ��ʽΪCH��CH��
��4����Է�������Ϊ72�ҷе���͵������Ľṹ��ʽ
 ��
����5��
 �����ƣ�ϵͳ��������2��3-�������飮
�����ƣ�ϵͳ��������2��3-�������飮
���� ��1����ϩ������̼̼��˫��������̼ʣ��ۼ���H���ͣ��ɴ�д������ʽ��
��2������CԪ�ء�HԪ���غ�ȷ�������ķ���ʽΪC6H12���ڴ�����������H2�����ӳɷ�Ӧ������2��2-�������飬������Ľṹ��ʽΪ��CH3��3C-CH=CH2��
��3����1mol��2mol HCl��ȫ�ӳɣ������������2��˫����1��������1mol�ȴ����ܺ�4mol����������ȫȡ����Ӧ�����ȴ����������4��Hԭ�ӣ�����ԭ����������2��Hԭ�ӣ��ݴ�ȷ����
��4����Է�������Ϊ72������������ͨʽΪCnH2n+2��12n+2n+2=72������õ�n=5������ʽΪ��C5H12��
�����ͬ���칹����CH3-CH2-CH2-CH2-CH3�� ��
�� �����Է��ӵ��۷е���ڷǼ��Է��ӣ��ݴ˽��з�����
�����Է��ӵ��۷е���ڷǼ��Է��ӣ��ݴ˽��з�����
��5���ж��л���������Ƿ���ȷ����л�������������������ȷ���������淶����������ԭ��
�ٳ�-----ѡ�̼��Ϊ������
�ڶ�-----���ȳ�̼��ʱ��֧�����Ϊ������
�۽�-----��֧�����һ�˱�ţ�
��С-----֧�����֮����С��������ṹ��ʽ�����Ҷ˻���˿��������ϡ���-----��֧�����һ�˱�š���ԭ��
�ݼ�-----��ȡ���������������˵Ⱦ���ʱ���Ӽ�ȡ������ʼ��ţ���ȡ������ͬ���ͰѼ�д��ǰ�棬���ӵ�д�ں��森
�л����������дҪ�淶�����ڽṹ�к��б����ģ�����ʱ�������α��������Ҳ���Ը��������λ�ã��á��ڡ������䡱�����ԡ��������������й����ŵ��л�������ʱ��Ҫѡ�������ŵ��̼����Ϊ�����������ŵ�λ����С��
��� �⣺��1����ϩ������̼̼��˫��������̼ʣ��ۼ���H���ͣ��ɴ�д������ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��2��n��������n��C����n��H��=n��������n��CO2����2n��H2O��=0.1mol��0.6mol��0.6mol��2=1��6��12����1�������к���6��Cԭ�ӡ�12��Hԭ�ӣ��ʸ����ķ���ʽΪC6H12���ڴ�����������H2�����ӳɷ�Ӧ������2.2-�������飬������Ľṹ��ʽΪ��CH3��3C-CH=CH2��
�ʴ�Ϊ����CH3��3C-CH=CH2��
��3����1mol��2mol HCl��ȫ�ӳɣ������������2��˫����1��������1mol�ȴ����ܺ�4mol����������ȫȡ����Ӧ�����ȴ����������4��Hԭ�ӣ��ȴ����������2��Hԭ���������Ȼ���ӳ�����ģ�����ԭ����������2��Hԭ�ӣ��ʸ���ΪCH��CH��
�ʴ�Ϊ��CH��CH��
��4����Է�������Ϊ72������������ͨʽΪCnH2n+2��12n+2n+2=72������õ�n=5������ʽΪ��C5H12��
�����ͬ���칹����CH3-CH2-CH2-CH2-CH3�� ��
�� �����Է��ӵ��۷е���ڷǼ��Է��ӣ����۷е���С��Ϊ
�����Է��ӵ��۷е���ڷǼ��Է��ӣ����۷е���С��Ϊ ��
��
�ʴ�Ϊ�� ��
��
��5������������ѡ����Ϊ5��������ȡ��������һ�˱�ţ�д����Ϊ��2��3-�������飬
�ʴ�Ϊ��2��3-�������飮
���� ���⿼���л�����ƶϣ�����ʽ����д��������ͬ���칹��ķ����жϣ��Ѷ��еȣ����ջ����ǽ���ؼ���

 ���ſ����ϵ�д�
���ſ����ϵ�д� ���Ŀ����ϵ�д�
���Ŀ����ϵ�д� ������ӱ������ͯ������ϵ�д�
������ӱ������ͯ������ϵ�д�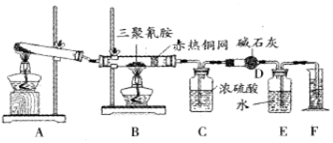
��1��д��Aװ���з�����Ӧ�Ļ�ѧ����ʽ��2KClO3$\frac{\underline{\;MnO_{2}\;}}{��}$2KCl+3O2����2KMnO4$\frac{\underline{\;��\;}}{\;}$K2MnO4+MnO2+O2����
��2��Cװ���ܲ�����Dװ�û��������ܣ�����ܡ����ܡ�����������Ũ������ˮ����ʯ�����ն�����̼��������λ�ã����ʯ�һ�ͬʱ����ˮ�Ͷ�����̼������ʵ��ʧ��
��3����Bװ���з�Ӧ��ȫ������ȡF��ˮ�������ʵ�����˳��Ϊ�ڢۢ٣�����ţ���
�ٶ��� ����ȴ������ �۵�ƽE��Fװ����Һ��
��4���ⶨ���������
| ���� | C | D |
| ʵ��ǰ | 101.0g | 56.0g |
| ����� | 106.4g | 69.2g |
����������ʵ�����ݣ�ͨ�������֪�����谷��ʵ��ʽΪCN2H2��
�������谷�ķ���ʽΪC3N6H6��
����װ����û��ͭ������Բⶨ�����Ӱ���Dzⶨ���÷���ʽ�ĵ�ԭ����ƫ��̼����ԭ����ƫС��
��5����֪���ᣨHCN���Ľṹ��ʽΪH-C��N���谷�Ľṹ��ʽΪH2NһC��N�������谷������ÿ��ԭ�ӵ�������������Ϊ8��2������ṹ��ʽΪ
 ��
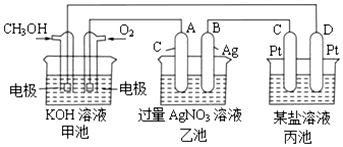
�� 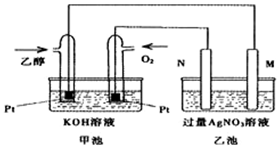 ��֪�״����Ҵ�������Ҫ���л�����ԭ�ϣ��ش��������⣺
��֪�״����Ҵ�������Ҫ���л�����ԭ�ϣ��ش��������⣺��1��ͼ��һ���Ҵ�ȼ�ϵ�س��¹���ԭ��ʾ��ͼ���ҳ��е������缫һ����ʯī�缫��һ�������缫������ʱM��N�����缫�������������٣�������˵����ȷ����C��
A��M�缫�IJ�����ʯī
B�����ҳ���ijһ�缫��������4.32gʱ����������������Ϊ448mL
C���ڴ˹����У��׳���OH-��ͨ�Ҵ���һ���ƶ�
D���ڴ˹����У��ҳ���Һ�е��Ӵ�M�缫��N�缫�ƶ�
��2��д���Ҵ�ȼ�ϵ�������ĵ缫��ӦʽO2+2H2O+4e-=4OH-��
��3����֪���״���ˮ��Ӧ ��2CH3OH��g��=CH3OCH3��g��+H2O��g��?��H1
�״���ϩ����Ӧ ��2CH3OH��g��=C2H4 ��g��+2H2O��g��?��H2
�Ҵ��칹����Ӧ ��CH3CH2OH��g��=CH3OCH3��g����?��H3
����ϩ����ֱ��ˮ�Ϸ�ӦC2H4 ��g��+H2O��g��=C2H5OH��g����?��H=��H1-��H2-��H3���ú���H1����H2����H3��ʾ����
��4����ҵ�Ͽ�����CO��CO2�������״����״��Ʊ��������Ϣ�����
| ��ѧ��Ӧ��ƽ�ⳣ�� | ƽ�ⳣ����ֵ | ||
| 500�� | 800�� | ||
| ��2H2��g��+CO��g��?CH3OH��g�� | K1 | 2.5 | 0.15 |
| ��H2��g��+CO2��g��?H2O��g��+CO��g�� | K2 | 1.0 | 2.50 |
| ��3H2��g��+CO2��g��?CH3OH��g��+H2O��g�� | K3 | 2.5 | 0.375 |
�ڷ�Ӧ�������ȷ�Ӧ��ѡ����ȡ������ȡ�����
| A�� | ������ڵ�AlCl3���������� | |
| B�� | ��Cl2��HCl�������ͨ������ʳ��ˮ�ɵõ�������Cl2 | |
| C�� | ��Ba��OH��2��Һ�ɼ���NaCl��AlCl3��NH4Cl��Na2SO4������Һ | |
| D�� | �÷�Һ©����ʳ���з�������� |
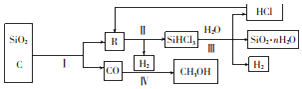
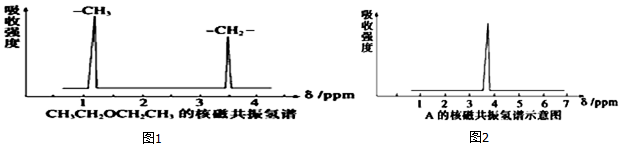
 ����˴Ź�����������2���źţ��μ�ͼ1����
����˴Ź�����������2���źţ��μ�ͼ1����