��Ŀ����
11��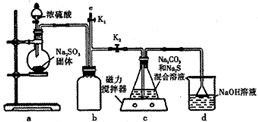 ���ۡ�8.12����ը�¹��У���ը��������軯��й©������ͨ������˫��ˮ�������������Һ���������Լ�����Ⱦ��
���ۡ�8.12����ը�¹��У���ը��������軯��й©������ͨ������˫��ˮ�������������Һ���������Լ�����Ⱦ�����ϣ��軯�ƻ�ѧʽNaCN��CԪ��+2�ۣ�NԪ��-3�ۣ�����ɫ�ᾧ�������׳��⡢�����Ŀ�������ζ���綾��������ˮ����ˮ�������軯�⡢ˮ��Һ�ʼ��ԣ�
��1���軯�ƣ�NaCN����Һ�ʼ��ԣ���ԭ����CN-+H2O?HCN+OH-�������ӷ���ʽ���ͣ���
��2����8.12����ը�¹ʺ���������֯Ⱥ����˫��ˮ��������˫��ˮ����NaCN����һ����ʽ�κ�һ����ʹʪ���ɫʯ����ֽ���������壬д���÷�Ӧ�Ļ�ѧ����ʽNaCN+H2O2+H2O=NaHCO3+NH3����
ij��ѧ��ȤС����ʵ�����Ʊ���������ƣ�������������������Һ��������軯�Ʒ�ˮ�ܷ����ŷţ�
��ʵ��һ��ʵ����ͨ����ͼ��ʾװ���Ʊ�Na2S2O3��
��3��ͼaװ����ʢװNa2SO3���������������Բ����ƿ��bװ�õ������ǰ�ȫƿ����ֹ������
��4��װ��c�еIJ�����Na2S2O3��C02����ȣ�ʵ�������װ��d�е�������NaOH��Na2CO3����������Na2SO3��
��5��ʵ���������e���������ʢNaOH��Һ��ѡ�NaOH��Һ������ˮ������CCl4������һ�֣���ע�������ٹر�K2��K1����ֹ���װ��ʱ��Ⱦ������
��ʵ������ⶨ�������������Һ������ķ�ˮ���軯�Ƶĺ�������֪��
�ٷ�ˮ���軯�Ƶ�����ŷű�Ϊ0.50mg/L��
��Ag++2CN-�T[Ag��CN��2]-��Ag++I-�TAgI����AgI�ʻ�ɫ����CN -������Ag+��Ӧ��
ʵ�����£�
ȡ25.00mL��������軯�Ʒ�ˮ����ƿ�в��μӼ���KI��Һ��ָʾ������1.000��10-4mol/L�ı�AgNO3��Һ�ζ�������AgNO3��Һ�����Ϊ2.50mL��
��6���ζ��յ���жϷ����ǵ������һ����������Һ�����ֵ���ɫ����
��7��������ķ�ˮ���軯�Ƶĺ���Ϊ0.98mg/L��
���� ��1��NaCNΪǿ�������Σ�ˮ��ʼ��ԣ�
��2�������£��軯���������������Һ��Ӧ��������ʹʪ��ĺ�ɫʯ����ֽ����ɫ�����壬������Ϊ���������ɵ���ʽ��Ϊ̼�����ƣ���ƽ��д����ʽ��
ʵ��һ��aװ���Ʊ���������cװ�����Ʊ�Na2S2O3����Ӧ����װ������ѹ��С��bΪ��ȫƿ���ã���ֹ��Һ������dװ�����ն���Ķ�������ֹ��Ⱦ������
��3���������ṹ��������֪ʢװNa2SO3���������ΪԲ����ƿ��bװ��Ϊ��ȫƿ��
��4��dװ�����ն�������d��������NaOH��Na2CO3������������������������ɣ�
��5���������װ��b�л��в����Ķ�������Ϊ��ֹ��Ⱦ������Ӧ������������Һ���գ�
��6��Ag+��CN-��Ӧ����[Ag��CN��2]-����CN-��Ӧ����ʱ��Ag+��I-����AgI��ɫ������˵����Ӧ����ζ��յ㣻
��7�������������������ʵ������ٸ��ݷ���ʽAg++2CN-=[Ag��CN��2]-������軯�Ƶĺ�����
��� �⣺��1��NaCNΪǿ�������Σ�ˮ��ʼ��ԣ���Ӧ�����ӷ���ʽΪ��CN-+H2O?HCN+OH-��
�ʴ�Ϊ��CN-+H2O?HCN+OH-��
��2�������£��軯���������������Һ��Ӧ��������ʹʪ��ĺ�ɫʯ����ֽ����ɫ�����壬������Ϊ���������ɵ���ʽ��Ϊ̼�����ƣ���Ӧ����ʽΪ��NaCN+H2O2+H2O=NaHCO3+NH3����
�ʴ�Ϊ��NaCN+H2O2+H2O=NaHCO3+NH3����
ʵ��һ��aװ���Ʊ���������cװ�����Ʊ�Na2S2O3����Ӧ����װ������ѹ��С��bΪ��ȫƿ���ã���ֹ��Һ������dװ�����ն���Ķ�������ֹ��Ⱦ������
��3���������ṹ��������֪ʢװNa2SO3���������ΪԲ����ƿ��bװ��Ϊ��ȫƿ����ֹ������
�ʴ�Ϊ��Բ����ƿ����ȫƿ����ֹ������
��4��dװ�����ն�������d��������NaOH��Na2CO3������������������������ɣ�
�ʴ�Ϊ��Na2SO3��
��5���������װ��b�л��в����Ķ�������Ϊ��ֹ��Ⱦ������Ӧ������������Һ���գ�
�ʴ�Ϊ��NaOH��Һ��
��6��Ag+��CN-��Ӧ����[Ag��CN��2]-����CN-��Ӧ����ʱ���������һ����������Һ��Ag+��I-����AgI��ɫ������˵����Ӧ����ζ��յ㣬
�ʴ�Ϊ���������һ����������Һ�����ֵ���ɫ������
��7������AgNO3�����ʵ���Ϊ2.5��10-3L��0.0001mol/L=2.50��10-7mol��
���ݷ���ʽAg++2CN-=[Ag��CN��2]-�������ķ�ˮ���軯�Ƶ�����Ϊ2.50��10-7mol��2��49g/mol=2.45��10-5g����ˮ���軯�Ƶĺ���Ϊ$\frac{2.45��1{0}^{-2}mg}{0.025L}$=0.98mg/L��
�ʴ�Ϊ��0.98��
���� ���⿼�������Ʊ�ʵ�顢���ʺ����ⶨ�ȣ���Ŀ�ۺ��Խ�ǿ���ؼ��Ƕ�ԭ�������⣬ע���Ʊ������뻷����ʶ����������Ԫ�ػ�����֪ʶ��ʵ���Ʊ�����ԭ����Ŀ�Ѷ��еȣ�

| A�� | ���з�̪��Na2CO3��Һ�м���BaCl2��Һ����ɫ��ȥ��֤��BaCl2��Һ������ | |
| B�� | ��������Һ������ԣ����ηֳ���ʽ�Ρ����κͼ�ʽ�� | |
| C�� | ��ĭ�������������������Һ��̼������Һ��Ӧ��������CO2��Al��OH��3��� | |
| D�� | NH4F��Һ�к���������HF�����NH4F��Һ���ܴ���ڲ����Լ�ƿ�� |
| A�� | ������ΪNA��CO��C2H4�����������ԼΪ22.4 L | |
| B�� | ��״���£�4.48 L��ˮ��D2O���к��е�������Ϊ2NA | |
| C�� | ��MnO2��Ũ������ȡCl2ʱ��ÿ����0.5mol Cl2��ת�Ƶ�����ΪNA | |
| D�� | 0.1 L 3.0 mol•L-1��NH4NO3��Һ�к���NH${\;}_{4}^{+}$����ĿΪ0.3NA |
| A�� | ���ǵķ�����֮��Ϊ1��2 | |
| B�� | ���ǵ�������֮��Ϊ5��8 | |
| C�� | ���ǵĵ�����֮��Ϊ1��2 | |
| D�� | �ֱ���1mol�Ʒ�Ӧ�����������������Ϊ1��1 |
ʵ�����ƣ���ˮ������ʵ��
ʵ��Ŀ�ģ���֤��ˮ���������ԣ�����Ư���ԣ������ԣ����۴���Cl-
ʵ����Ʒ����Ҫ������Ʒ���Թܡ���ͷ�ιܣ�
ѡ���Լ������Ƶı�����ˮ��NaOH��Һ�����з�̪��NaOH��Һ�����������������Լ���Ʒ����Һ����������Һ��ϡ���ᣮ
ʵ�鲽�裺������֤��ˮ���������ѧ���ʣ�
ʵ���¼����
| ������ | �������� | ʵ������ | ʵ����� |
| �� | ��ˮ������ | ||
| �� | ��ˮ��Ư���� | ||
| �� | ��ˮ�д���Cl- |
| ������ | H+��K+��Al3+��NH4+��Mg2+ |
| ������ | Cl-��Br-��OH-��CO32-��AlO2- |

��1������Һ��һ�����е���������H+��Al3+��NH4+��Mg2+�����Ӧ���ʵ���Ũ��֮��Ϊ2��2��2��3����Һ��һ�������ڵ���������OH-��CO32-��AlO2-��
��2��a=1��b=7��c=9��
��3��д���������ӷ���ʽ��
AB��2Na2O2+2H2O=4Na++4OH-+O2����NH4++OH-$\frac{\underline{\;\;��\;\;}}{\;}$NH3+H2O
CD��Al��OH��3+OH-=AlO2-+2H2O��
| A�� | Fe2+��Na+��NO3-��Cl- | B�� | Ba2+��Na+��NO3-��Cl- | ||
| C�� | SO42-��SO32-��NH4+��Na+ | D�� | Mg2+��Na+��Br-��AlO2- |
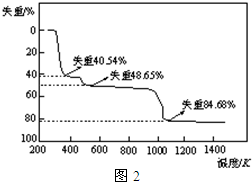 ��5��ȡ��������������������ط��������ȷֽ���Ҫ��Ϊ�����Σ�323K-523K��553K-687K��1043K���ϲ���ʧ�أ����ȷֽ��TG����ͼ2����֪��ʧ��%=$\frac{���ȼ��ٵ�����}{ԭ������Ʒ��������}$��100%������ͼʾ���ݼ���ȷ��ÿ���ֽ�IJ��д����һ�ηֽ����Ļ�ѧʽAl2��SO4��3.3H2O�������η�Ӧ��ѧ����ʽAl2��SO4��3$\frac{\underline{\;\;��\;\;}}{\;}$Al2O3+3SO3����
��5��ȡ��������������������ط��������ȷֽ���Ҫ��Ϊ�����Σ�323K-523K��553K-687K��1043K���ϲ���ʧ�أ����ȷֽ��TG����ͼ2����֪��ʧ��%=$\frac{���ȼ��ٵ�����}{ԭ������Ʒ��������}$��100%������ͼʾ���ݼ���ȷ��ÿ���ֽ�IJ��д����һ�ηֽ����Ļ�ѧʽAl2��SO4��3.3H2O�������η�Ӧ��ѧ����ʽAl2��SO4��3$\frac{\underline{\;\;��\;\;}}{\;}$Al2O3+3SO3����