��Ŀ����
�л������һ�����ϣ���ϳ�·����ͼ��������������-CH3������Է�������ͨ���������Ϊ88�����ĺ˴Ź���������ʾֻ������壻�����պ��㴼��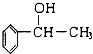 ����Ϊͬϵ�
����Ϊͬϵ�
��֪��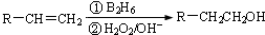
��1������ϵͳ��������A�������� ��
��2��C������Cu��OH��2����Һ��Ӧ�Ļ�ѧ����ʽΪ ��
��3������ֻ������-CH3��D�ܷ���������Ӧ���ڴ���������1mol D��2mol H2���Է�Ӧ�����ң���D�Ľṹ��ʽΪ ��
��4�������ҷ�Ӧ�Ļ�ѧ����ʽΪ ��
��5��д�����������������ҵ�ͬ���칹��Ľṹ��ʽ�� ��
�ٱ�������3��ȡ�����������
���������ԣ���Fe3+����ɫ
�۱����� 3��ȡ����������Ż������ڣ�
 ����Ϊͬϵ�
����Ϊͬϵ�
��֪��
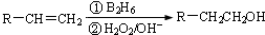
��1������ϵͳ��������A��������
��2��C������Cu��OH��2����Һ��Ӧ�Ļ�ѧ����ʽΪ
��3������ֻ������-CH3��D�ܷ���������Ӧ���ڴ���������1mol D��2mol H2���Է�Ӧ�����ң���D�Ľṹ��ʽΪ
��4�������ҷ�Ӧ�Ļ�ѧ����ʽΪ
��5��д�����������������ҵ�ͬ���칹��Ľṹ��ʽ��
�ٱ�������3��ȡ�����������
���������ԣ���Fe3+����ɫ
�۱����� 3��ȡ����������Ż������ڣ�
���㣺�л�����ƶ�
ר�⣺
�����������պ��㴼��Ϊͬϵ�����Ϊ������Ϊ���ᣬ���߷���������Ӧ���ɱ����ɱ��ķ���ʽ��֪�������к���1��-COOH������Է�������ͨ���������Ϊ88��ȥ��1���Ȼ���ʣ������ʽ��Ϊ88-45=43��������Ϊ-C3H7����ķ���ʽΪC4H8O2��������������-CH3�����Ϊ��CH3��2CHCOOH�����ƿ�֪CΪ��CH3��2CHCHO��BΪ��CH3��2CHCH2OH����Ϸ�Ӧ��Ϣ��֪AΪ��CH3��2C=CH2���ɼס�������ʽ��֪�����ҷ���ʽΪC9H12O����3���б��к�������-CH3�����ڼ��к���2�����������в����ڼ�������Ϊ �����Ϊ
�����Ϊ ��D�ɷ���������Ӧ���ڴ���������1mol D��2mol H2���Է�Ӧ�����ң�����DΪC9H8O����D�Ľṹ��ʽΪ
��D�ɷ���������Ӧ���ڴ���������1mol D��2mol H2���Է�Ӧ�����ң�����DΪC9H8O����D�Ľṹ��ʽΪ ���ݴ˽��
���ݴ˽��
 �����Ϊ
�����Ϊ ��D�ɷ���������Ӧ���ڴ���������1mol D��2mol H2���Է�Ӧ�����ң�����DΪC9H8O����D�Ľṹ��ʽΪ
��D�ɷ���������Ӧ���ڴ���������1mol D��2mol H2���Է�Ӧ�����ң�����DΪC9H8O����D�Ľṹ��ʽΪ ���ݴ˽��
���ݴ˽�����
�⣺�����պ��㴼��Ϊͬϵ�����Ϊ������Ϊ���ᣬ���߷���������Ӧ���ɱ����ɱ��ķ���ʽ��֪�������к���1��-COOH������Է�������ͨ���������Ϊ88��ȥ��1���Ȼ���ʣ������ʽ��Ϊ88-45=43��������Ϊ-C3H7����ķ���ʽΪC4H8O2��������������-CH3�����Ϊ��CH3��2CHCOOH�����ƿ�֪CΪ��CH3��2CHCHO��BΪ��CH3��2CHCH2OH����Ϸ�Ӧ��Ϣ��֪AΪ��CH3��2C=CH2���ɼס�������ʽ��֪�����ҷ���ʽΪC9H12O����3���б��к�������-CH3�����ڼ��к���2�����������в����ڼ�������Ϊ �����Ϊ
�����Ϊ ��D�ɷ���������Ӧ���ڴ���������1mol D��2mol H2���Է�Ӧ�����ң�����DΪC9H8O����D�Ľṹ��ʽΪ
��D�ɷ���������Ӧ���ڴ���������1mol D��2mol H2���Է�Ӧ�����ң�����DΪC9H8O����D�Ľṹ��ʽΪ ��
��
��1��������������֪��AΪ��CH3��2C=CH2������ϵͳ��������A�������ǣ�2-����ϩ���ʴ�Ϊ��2-����ϩ��
��2��C������Cu��OH��2����Һ��Ӧ�Ļ�ѧ����ʽΪ����CH3��2CHCHO+2Cu��OH��2
��CH3��2CHCOOH+Cu2O��+2H2O��
�ʴ�Ϊ����CH3��2CHCHO+2Cu��OH��2
��CH3��2CHCOOH+Cu2O��+2H2O��
��3��������������֪��D�Ľṹ��ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��4�������ҷ�Ӧ�Ļ�ѧ����ʽΪ����CH3��2CHCOOH+
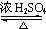
 +H2O��
+H2O��
�ʴ�Ϊ����CH3��2CHCOOH+
 +H2O��
+H2O��
��5���ҵĽṹ��ʽΪ ����ͬ���칹����ϣ���������3��ȡ����������ţ��������ԣ������Ϊ-OH��-CH3��-CH2CH3�������� 3��ȡ����������Ż������ڣ����ͬ���칹��Ϊ
����ͬ���칹����ϣ���������3��ȡ����������ţ��������ԣ������Ϊ-OH��-CH3��-CH2CH3�������� 3��ȡ����������Ż������ڣ����ͬ���칹��Ϊ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
 �����Ϊ
�����Ϊ ��D�ɷ���������Ӧ���ڴ���������1mol D��2mol H2���Է�Ӧ�����ң�����DΪC9H8O����D�Ľṹ��ʽΪ
��D�ɷ���������Ӧ���ڴ���������1mol D��2mol H2���Է�Ӧ�����ң�����DΪC9H8O����D�Ľṹ��ʽΪ ��
����1��������������֪��AΪ��CH3��2C=CH2������ϵͳ��������A�������ǣ�2-����ϩ���ʴ�Ϊ��2-����ϩ��
��2��C������Cu��OH��2����Һ��Ӧ�Ļ�ѧ����ʽΪ����CH3��2CHCHO+2Cu��OH��2
| �� |
�ʴ�Ϊ����CH3��2CHCHO+2Cu��OH��2
| �� |
��3��������������֪��D�Ľṹ��ʽΪ
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
����4�������ҷ�Ӧ�Ļ�ѧ����ʽΪ����CH3��2CHCOOH+

| Ũ����� |
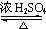
 +H2O��
+H2O���ʴ�Ϊ����CH3��2CHCOOH+

| Ũ���� |
| �� |
 +H2O��
+H2O����5���ҵĽṹ��ʽΪ
 ����ͬ���칹����ϣ���������3��ȡ����������ţ��������ԣ������Ϊ-OH��-CH3��-CH2CH3�������� 3��ȡ����������Ż������ڣ����ͬ���칹��Ϊ
����ͬ���칹����ϣ���������3��ȡ����������ţ��������ԣ������Ϊ-OH��-CH3��-CH2CH3�������� 3��ȡ����������Ż������ڣ����ͬ���칹��Ϊ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
���������⿼���л�����ƶϣ�����ȷ���Ľṹ�ǹؼ�����������л���Ľṹ�����ʽ�����ƶϣ���Ҫ�������չ����ŵ�������ת����ע�����ò��෨ȷ��A�ķ���ʽ����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
ƫ�����£�C2H8N2����һ�ָ���ȼ�ϣ�ȼ�ղ���������������Ϊ�������ػ�����ƶ�����������������ȷ���ǣ�������
| A��ƫ�����µ�Ħ������Ϊ60 g?mol-1 |
| B��6.02��1023��ƫ�����·��ӵ�����Ϊ60 |
| C��1 molƫ�����µ�����Ϊ60 g?mol-1 |
| D��6 gƫ�����º���NA��ƫ�����·��� |
�������ӷ���ʽ��д��ȷ���ǣ�������
| A��������ϡ���ᷴӦ��2Fe+6H+=2Fe3++3H2�� |
| B�������е���̼������Һ��CO32-+2H+=H2O+CO2�� |
| C�������е�������������Һ��HCl+OH-=H2O+Cl- |
| D��ϡ�������ʯ��ʯ�ϣ�CO32-+2H+=H2O+CO2�� |
�������и������ӻ���ӵ���Һ�У�ͨ�����SO2��������ܴ���������ǣ�������
| A��H+��Ca2+��Fe3+��NO3- |
| B��Ba2+��Cl-��Al3+��H+ |
| C��Na+��NH4+��I-��HS- |
| D��Na+��NH3?H2O��K+��Cl- |
ij����������ܺ���NH4Cl��NaOH��KOH��AgNO3��A1C13�е������֣�������ˮ��ֽ��裬�õ���ɫ������ҺA��ȡA��������ʵ�飺��������ʵ�����������Ʋ���ȷ���ǣ�������
��պȡA���գ�δ��������ֻ�ɫ��
��ȡA��μ���ϡ�������������������ɰ�ɫ��������������ܽ⣻
��ȡ1mLA����3����ȩ��ˮԡ���ȣ��Թ��ڱڳ��ֹ����ġ���������
��պȡA���գ�δ��������ֻ�ɫ��
��ȡA��μ���ϡ�������������������ɰ�ɫ��������������ܽ⣻
��ȡ1mLA����3����ȩ��ˮԡ���ȣ��Թ��ڱڳ��ֹ����ġ���������
| A��A���A1O2- |
| B��A��c��C1-����c��K+�� |
| C��A��pH��7 |
| D��������NH4Cl��KOH��AgNO3 ��� |