��Ŀ����
�������£���a mol N2��b mol H2�Ļ������ͨ��һ���̶��ݻ����ܱ������У��������·�Ӧ��N2��g��+3H2��g��?2NH3��g��
��1������Ӧ���е�ijʱ��tʱ��n��N2��=13mol��n��NH3��=6mol������a��ֵ ��
��2����Ӧ��ƽ��ʱ�������������Ϊ716.8L����״���£�������NH3�ĺ��������������Ϊ25%����ԭ���������ƽ���������ѹǿ֮��P��ʼ����P��ƽ��= ����д����������ȣ���ͬ����
��3��ԭ��������У�a��b= ��
��4���ﵽƽ��ʱ��N2��H2��ת����֮�ȣ�a��N2����a ��H2��= ��
��5��ƽ���������У�n��N2����n��H2����n��NH3��= ��
��1������Ӧ���е�ijʱ��tʱ��n��N2��=13mol��n��NH3��=6mol������a��ֵ
��2����Ӧ��ƽ��ʱ�������������Ϊ716.8L����״���£�������NH3�ĺ��������������Ϊ25%����ԭ���������ƽ���������ѹǿ֮��P��ʼ����P��ƽ��=
��3��ԭ��������У�a��b=
��4���ﵽƽ��ʱ��N2��H2��ת����֮�ȣ�a��N2����a ��H2��=
��5��ƽ���������У�n��N2����n��H2����n��NH3��=
���㣺��ѧƽ���Ӱ������,��ѧƽ��ļ���
ר�⣺��ѧƽ��ר��
��������1���������ɰ��������ʵ�������������ʽ���㣻
��2�����������������У������ѹǿ֮�ȵ������ʵ���֮�ȣ������Ӧǰ��ƽ��ʱ��������ʵ������ɣ�
��3���������������ܵ����ʵ��������ã�2������ab�����ʵ�������õ���
��4���ɣ�3����֪����ʼ�����������ʵ���Ϊ24mol��ƽ��ʱ�μӷ�Ӧ�ĵ��������ʵ���Ϊ4mol���μӷ�Ӧ�����������ʵ���Ϊ12mol��������ﵽƽ��ʱ��N2��H2��ת���ʣ��ݴ˼��㣻
��5������ǰ��������ݼ�����ﵽƽ��ʱ����Ӧ��������ֵ����ʵ������ݴ˼��㣮
��2�����������������У������ѹǿ֮�ȵ������ʵ���֮�ȣ������Ӧǰ��ƽ��ʱ��������ʵ������ɣ�
��3���������������ܵ����ʵ��������ã�2������ab�����ʵ�������õ���
��4���ɣ�3����֪����ʼ�����������ʵ���Ϊ24mol��ƽ��ʱ�μӷ�Ӧ�ĵ��������ʵ���Ϊ4mol���μӷ�Ӧ�����������ʵ���Ϊ12mol��������ﵽƽ��ʱ��N2��H2��ת���ʣ��ݴ˼��㣻
��5������ǰ��������ݼ�����ﵽƽ��ʱ����Ӧ��������ֵ����ʵ������ݴ˼��㣮
���
�⣺��1�����ݷ���ʽ���㣺
N2 ��g��+3H2��g��
2NH3��g����
��ʼ��mol����a b 0
ת����mol����3 9 6
tʱ�̣�mol����a-3 b-9 6
����a-3=13����a=16��
�ʴ�Ϊ��16��
��2����Ӧ��ƽ��ʱ���������Ϊ
=32mol�����ò��������㣺
N2 ��g��+3H2��g��
2NH3��g�������ʵ������١�n
1mol 3mol 2mol 2mol
ת����4mol 12mol 8mol 8mol
��ԭ�������Ϊ32mol+8mol=40mol��
P��ʼ����P��ƽ��=n��ʼ����n��ƽ��=40��32=5��4���ʴ�Ϊ��5��4��
��3�����ò��������㣺
N2 ��g��+3H2��g��
2NH3��g�������ʵ������١�n
1mol 3mol 2mol 2mol
ת����4mol 12mol 8mol 8mol
��ԭ�������Ϊ32mol+8mol=40mol��
�ɣ�1��֪a=16mol����b=40mol-16mol=24mol��
���ԣ�a��b=16mol��24mol=2��3��
�ʴ�Ϊ��2��3��
��4���ɣ�3����֪����ʼ�����������ʵ���Ϊ24mol��
ƽ��ʱ�μӷ�Ӧ�ĵ��������ʵ���Ϊ4mol���μӷ�Ӧ�����������ʵ���Ϊ12mol��
���Դﵽƽ��ʱ��N2��H2��ת����֮�Ȧ���N2��������H2��=
��
=1��2��
�ʴ�Ϊ��1��2��
��5��ƽ�����������Ϊ��N2Ϊ16mol-4mol=12 mol��NH3Ϊ8 mol��H2Ϊ32mol-12mol-8mol=12mol��
ƽ���������У�n��N2����n��H2����n��NH3��=12mol��12mol��8mol=3��3��2��
�ʴ�Ϊ��3��3��2��
N2 ��g��+3H2��g��
| ���¡���ѹ |
| ���� |
��ʼ��mol����a b 0
ת����mol����3 9 6
tʱ�̣�mol����a-3 b-9 6
����a-3=13����a=16��
�ʴ�Ϊ��16��
��2����Ӧ��ƽ��ʱ���������Ϊ
| 716.8L |
| 22.4mol?L-1 |
N2 ��g��+3H2��g��
| ���¸�ѹ |
| ���� |
1mol 3mol 2mol 2mol
ת����4mol 12mol 8mol 8mol
��ԭ�������Ϊ32mol+8mol=40mol��
P��ʼ����P��ƽ��=n��ʼ����n��ƽ��=40��32=5��4���ʴ�Ϊ��5��4��
��3�����ò��������㣺
N2 ��g��+3H2��g��
| ���¸�ѹ |
| ���� |
1mol 3mol 2mol 2mol
ת����4mol 12mol 8mol 8mol
��ԭ�������Ϊ32mol+8mol=40mol��
�ɣ�1��֪a=16mol����b=40mol-16mol=24mol��
���ԣ�a��b=16mol��24mol=2��3��
�ʴ�Ϊ��2��3��
��4���ɣ�3����֪����ʼ�����������ʵ���Ϊ24mol��
ƽ��ʱ�μӷ�Ӧ�ĵ��������ʵ���Ϊ4mol���μӷ�Ӧ�����������ʵ���Ϊ12mol��
���Դﵽƽ��ʱ��N2��H2��ת����֮�Ȧ���N2��������H2��=
| 4mol |
| 16mol |
| 12mol |
| 24mol |
�ʴ�Ϊ��1��2��
��5��ƽ�����������Ϊ��N2Ϊ16mol-4mol=12 mol��NH3Ϊ8 mol��H2Ϊ32mol-12mol-8mol=12mol��
ƽ���������У�n��N2����n��H2����n��NH3��=12mol��12mol��8mol=3��3��2��
�ʴ�Ϊ��3��3��2��
���������⿼�黯ѧƽ��ļ��㣬��Ŀ�ѶȲ���ע����������ʽ����ϻ�ѧ����ʽ���㣮

��ϰ��ϵ�д�
�����Ŀ
 ��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺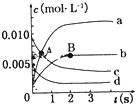 ��2L�ܱ������У�800��ʱ��Ӧ2NO��g��+O2��g��?2NO2��g����ϵ�У�n��NO����ʱ��ı仯�����
��2L�ܱ������У�800��ʱ��Ӧ2NO��g��+O2��g��?2NO2��g����ϵ�У�n��NO����ʱ��ı仯����� Al-Mg�ڲ�ͬ�ĵ������Һ�зֱ�ԭ���A��B����ͼ��ʾ��
Al-Mg�ڲ�ͬ�ĵ������Һ�зֱ�ԭ���A��B����ͼ��ʾ��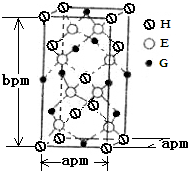 A��B��D��E��G��H����ǰ�����ڵ�Ԫ�أ�������ǰ�����ڵĻ�̬ԭ����A��δ�ɶԵ�����ࣻB��̬ԭ�ӵ�L���Ӳ��p�ܼ�����һ���չ����D �Ļ�̬ԭ�ӵ�2p�������1�����ӵ������������������ӵ����������෴��E�Ļ�̬ԭ��M����6���˶�״̬��ͬ�ĵ��ӣ� G�����ڱ���λ�ڵ�8�У�H��ԭ��������G��3��������Ϣ�ش��������⣺
A��B��D��E��G��H����ǰ�����ڵ�Ԫ�أ�������ǰ�����ڵĻ�̬ԭ����A��δ�ɶԵ�����ࣻB��̬ԭ�ӵ�L���Ӳ��p�ܼ�����һ���չ����D �Ļ�̬ԭ�ӵ�2p�������1�����ӵ������������������ӵ����������෴��E�Ļ�̬ԭ��M����6���˶�״̬��ͬ�ĵ��ӣ� G�����ڱ���λ�ڵ�8�У�H��ԭ��������G��3��������Ϣ�ش��������⣺