��Ŀ����
5��W��X��Y��ZΪ�������ڣ���ϡ������Ԫ���⣩������Ԫ�أ����ǵ�ԭ������������������ֻ��Y�ǽ���Ԫ�أ�X��Z����ͬһ�����Ԫ�أ�Y��������������W������������ȣ�Y��Z��Ԫ�ص�������֮��ΪW��X��Ԫ�ص�������֮�͵�3������1���ɴ˿�֪Ԫ��WΪ�⣻ Y Ϊ�ƣ�������Ԫ�����ƣ�
��2��Zλ��Ԫ�����ڱ��е������ڣ��ڢ�A��
��3��W�ֱ���X��Z���γ���Ӧ���⻯������ֻ�����ķе��ɸߵ��͵�˳��ΪH2O��H2S���÷���ʽ��ʾ��
��4��W��X��Y��Z����Ԫ�ؼ����ӵ����Ӱ뾶�ɴ�С��˳����S2-��O2-��Na+��H+��
��5������������Ԫ������η�Ӧ�����ӷ���ʽHSO3-+H+=SO2��+H2O
��6��Z�ĵͼ�������ͨ��Cl2���ʵ�ˮ��Һ�У�������Ӧ�Ļ�ѧ����ʽΪSO2+Cl2+2H2O�TH2SO4+2HCl��
���� W��X��Y��ZΪ�������ڣ���ϡ������Ԫ���⣩������Ԫ�أ����ǵ�ԭ��������������Y��������������W��������������ȣ�����ͬ���壬����ֻ��YΪ����Ԫ�أ���Yֻ�ܴ���IA����A�壬��YΪ��A�壬���ԭ��������֪WΪBԪ�أ�YΪAl����X��Z��ԭ��������Ϊa��b����13+b=3��a+5������b=3a+2������5��a����b��17��Z���Ƕ�����Ԫ�أ����������⣬��YΪIA�壬���ԭ��������֪WΪHԪ�أ�YΪNa����X��Z��ԭ��������Ϊa��b����11+b=3��a+1������b+8=3a����ԭ�����������ֻ��YΪ�������ɵ�5��a��10��14��b��17����14��3a-8��17����$\frac{22}{3}$��a��$\frac{25}{3}$����a=8����b=16����XΪOԪ�ء�ZΪSԪ�أ��ɴ˷������
��� �⣺W��X��Y��ZΪ�������ڣ���ϡ������Ԫ���⣩������Ԫ�أ����ǵ�ԭ��������������Y��������������W��������������ȣ�����ͬ���壬����ֻ��YΪ����Ԫ�أ���Yֻ�ܴ���IA����A�壬��YΪ��A�壬���ԭ��������֪WΪBԪ�أ�YΪAl����X��Z��ԭ��������Ϊa��b����13+b=3��a+5������b=3a+2������5��a����b��17��Z���Ƕ�����Ԫ�أ����������⣬��YΪIA�壬���ԭ��������֪WΪHԪ�أ�YΪNa����X��Z��ԭ��������Ϊa��b����11+b=3��a+1������b+8=3a����ԭ�����������ֻ��YΪ�������ɵ�5��a��10��14��b��17����14��3a-8��17����$\frac{22}{3}$��a��$\frac{25}{3}$����a=8����b=16����XΪOԪ�ء�ZΪSԪ�أ�
��1���ɴ˿�֪Ԫ��WΪ��Ԫ�أ�Y Ϊ��Ԫ�أ��ʴ�Ϊ���⣻�ƣ�
��2����Ԫ��Ϊ16��Ԫ�أ������ڱ��е�λ��Ϊ���������ڣ��ڢ�A�壬�ʴ�Ϊ��������A��
��3����Ԫ�طֱ�����Ԫ�ء���Ԫ�����γ���Ӧ���⻯����ˮ�����⣬ˮ���Ӽ����������е��쳣�ĸߣ����������ֻ�����ķе��ɸߵ��͵�˳��ΪH2O��H2S���ʴ�Ϊ��H2O��H2S��
��4�����Ӳ�Խ��뾶Խ���Ӳ�����ͬ���˵����Խ��뾶ԽС������H��O��Na��S����Ԫ�ؼ����ӵ����Ӱ뾶�ɴ�С��˳���ǣ�S2-��O2-��Na+��H+��
�ʴ�Ϊ��S2-��O2-��Na+��H+��
��5������������Ԫ������ηֱ������������ƺ��������Ʒ�Ӧ�����ӷ���ʽ��HSO3-+H+=SO2��+H2O���ʴ�Ϊ��HSO3-+H+=SO2��+H2O��
��6��S�ĵͼ��������Ƕ�������ͨ��Cl2���ʵ�ˮ��Һ�У���Ӧ�ķ���ʽΪ��SO2+Cl2+2H2O�TH2SO4+2HCl���ʴ�Ϊ��SO2+Cl2+2H2O�TH2SO4+2HCl��
���� ���⿼��ṹ����λ�ù�ϵӦ�ã����ؿ���ѧ���ķ��������������������ն�����Ԫ�ؼ�Ԫ�ػ�������ɵȣ�

| A�� | ������Ϊ35����ԭ�ӣ�${\;}_{17}^{35}$Cl | B�� | �Ȼ�淋ĵ���ʽ�� | ||
| C�� | ����Ľṹʽ��H-S-H | D�� | ��������ķ���ʽ��SiO2 |
| ����\�� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | O |
| 2 | E | |||||||
| 3 | A | C | D | G | ||||
| 4 | B | F |
��2��DԪ�ص�����������Ӧˮ�������������Ʒ�Ӧ�����ӷ���ʽ��Al��OH��3+OH-=AlO2-+2H2O
��3��A��B��C����Ԫ�ذ�ԭ�Ӱ뾶�ɴ�С��˳������ΪK��Na��Mg����Ԫ�ط��ţ�
��4��EԪ���⻯��Ľṹʽ��H-O-H�����⻯���ڳ����¸�B������Ӧ�Ļ�ѧ����ʽ��2K+2H2O=2KOH+H2����
��5��FԪ�ظ�AԪ���γɻ�����ĵ���ʽ��
 ���������ոû�����ʱ������ʻ�ɫ��
���������ոû�����ʱ������ʻ�ɫ����6��C��D�ĵ������缫��ϡ�������������Һ������ԭ��أ��为���ĵ缫��ӦʽΪ��Mg-2e-=Mg2+��


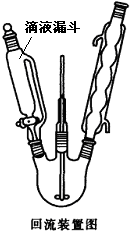 ������������Mr=128����һ����Ҫ��Ⱦ���м��壮����һ�ֻ�ɫ��״�ᾧ������ˮ�����¶������ܽ�����������Ҵ������ѡ��״���������������ѡ�ü�����������������������ѡ���Ի�ԭ��װ������ͼ��ʾ�����䷴Ӧʽ���£�
������������Mr=128����һ����Ҫ��Ⱦ���м��壮����һ�ֻ�ɫ��״�ᾧ������ˮ�����¶������ܽ�����������Ҵ������ѡ��״���������������ѡ�ü�����������������������ѡ���Ի�ԭ��װ������ͼ��ʾ�����䷴Ӧʽ���£�

