��Ŀ����
11��40��ʱ���ڰ�-ˮ��ϵ�в���ͨ��CO2���������ӵı仯������ͼ��ʾ������˵������ȷ���ǣ�������| A�� | ��pH=9.0ʱ��c��NH4+����c��HCO3-����c��NH2COO-����c��CO32-�� | |
| B�� | ��ͼ����CO2��ͨ�룬$\frac{{c��OH}^{-}��}{{c��NH}_{3}•{H}_{2}O��}$���ϼ�С | |
| C�� |  ����ͼ��ʾ��ͬpH����Һ��һ�����ڹ�ϵ��c��NH4+����2c��CO32-��+c��HCO3-��+c��NH2COO-�� | |
| D�� | ����ҺpH���Ͻ��͵Ĺ����У��к�NH2COO-���м�������� |
���� A������ͼ����pH=9.0ʱ�����������ж�����Ũ�ȴ�С��
B�����Ŷ�����̼��ͨ�룬��Һ��笠����ӵ�Ũ���������һˮ�ϰ��ĵ���ƽ�ⳣ������$\frac{{c��OH}^{-}��}{{c��NH}_{3}•{H}_{2}O��}$�ı仯��
C�����¶���pH=7ʱ�ʼ��ԣ���c��H+����c��OH-������ϵ���غ��жϣ�
D������ͼ�����߱仯��֪��ͨ�������̼��������NH2COO-���м�������ɣ�
��� �⣺A������ͼ���֪��pH=9.0ʱ��������Ũ�ȴ�СΪ��c��NH4+����c��HCO3-����c��NH2COO-����c��CO32-������A��ȷ��
B������һˮ�ϰ��ĵ���ƽ�ⳣ����֪��$\frac{{c��OH}^{-}��}{{c��NH}_{3}•{H}_{2}O��}$=$\frac{{K}_{b}}{c��N{{H}_{4}}^{+}��}$�����Ŷ�����̼��ͨ�룬��Һ��笠����ӵ�Ũ����������ñ�ֵ��С������ȷ��
C��c��NH4+��+c��H+��=c��OH-��+2c��CO32-��+c��HCO3-��+c��NH2COO-����40��ʱpH=7����Һ�ʼ��ԣ���c��H+����c��OH-������c��NH4+����2c��CO32-��+c��HCO3-��+c��NH2COO-������C����
D������ͼ���֪����ҺpHС��10.5���к�NH2COO-���м�������ɣ���D��ȷ��
��ѡC��
���� ���⿼��������Ũ�ȴ�С�Ƚϡ�����ϵĶ����жϵ�֪ʶ����Ŀ�Ѷ��еȣ���ȷͼ�����߱仯�ĺ���Ϊ���ؼ���ע�����յ���غ㡢�����غ㼰�ε�ˮ��ԭ�������ڷ���������������ѧ���ķ������������������Ӧ��������

��1���ڶ������У�Ԫ�صĵ�һ�����ܴ���B��N֮���Ԫ����3�֣�
��2��ijԪ��λ�ڵ������ڢ��壬���̬ԭ�ӵ�δ�ɶԵ��������̬̼ԭ�ӵ�δ�ɶԵ�������ͬ�������̬ԭ�ӵļ۲�����Ų�ʽΪ3d84s2��
��3����ϩͪ��CH2�TC�TO����һ����Ҫ���л��м��壬����CH3COOH�ڣ�C2H5O��3P�TO�����¼�����H2O�õ�����ϩͪ������̼ԭ���ӻ����������sp2��sp��1mol��C2H5O��3P�TO�����к��еĦҼ�����ĿΪ25NA��
��4����֪��̬NH3��H2O��HF��������ܺͽṹ��ͼ1��
| ���� | ���X-H��Y | ����kJ��mol-1 |
| ��HF��n | D-H��F | 28.1 |
| �� | O-H��O | 18.8 |
| ��NH3��n | N-H��N | 5.4 |
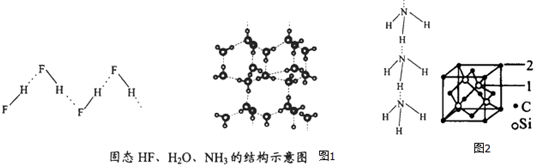
����H2O��HF��NH3�е����ν��͵�ԭ������ļ����ǣ�HF��n��������NH3��n����ƽ��ÿ�����Ӻ������������2������HF��n�ͣ�NH3��nֻ��1��������Ҫ�˷���������ܼ����DZ�����HF��n����NH3��n��
��5��̼����Ľṹ����ʯ���ƣ���Ӳ�Ƚ����ڽ��ʯ�����н�ǿ����ĥ���ܣ�̼���辧���ṹ��ÿ��̼ԭ����Χ�����������Ĺ�ԭ����4������̼ԭ�ӵȾ��������̼ԭ����12������֪̼���辧���߳�Ϊapm����ͼ2��1�Ź�ԭ�Ӻ�2��̼ԭ��֮��ľ���Ϊ$\frac{\sqrt{11}}{4}$apm��̼������ܶ�Ϊ$\frac{1.6��1{0}^{32}}{{a}^{3}{N}_{A}}$g/cm3��
 ijС��ͬѧͨ���Ա�ʵ���˽�Na2CO3��NaHCO3���ʵ���ͬ�����������д��ʵ�鱨���һ���֣�
ijС��ͬѧͨ���Ա�ʵ���˽�Na2CO3��NaHCO3���ʵ���ͬ�����������д��ʵ�鱨���һ���֣� | ʵ����� | ʵ�鲽�裨���ݣ� | ���� |
| �� | �����б�ǩa��b���Թ��зֱ����1.0gNa2CO3�����NaHCO3���壬�۲���ۣ� | / |
| �� | ���������Թ��зֱ����10.0mLˮ���������۲����� | / |
| �� | �ٷֱ������Թ��еμ�2�η�̪��Һ���۲����� | ��Һ��� |
| �� | ���ȴ��Թ�һ��ʱ�䣮��ע������մ����ˮ����ͭ��ĩ�� | ����ˮ����ͭ��ĩ�����������A�ձ���û����������B�ձ����а�ɫ�������� |
��2����֪��20��ʱNa2CO3���ܽ��Ϊ20.5g����ʵ��ڣ���ʵ����ţ������֪��20��ʱNaHCO3���ܽ��С��20.5g�������Na2CO3��Һ�в���ͨ��CO2���壬�����ǰ�ɫ������������ѧ����ʽ��Na2CO3+CO2+H2O=2NaHCO3����
��3����ʵ��ܿ��Եó�������̼���Ƶ��ȶ���ǿ��̼�����ƣ��û�ѧ����ʽ˵���ó����۵�ԭ��2NaHCO3$\frac{\underline{\;\;��\;\;}}{\;}$Na2CO3+CO2��+H2O��
| �� ���� | ��A | 0 | ||||||
| 1 | A | ��A | ��A | ��A | ��A | ��A | ��A | He |
| 2 | B | C | D | |||||
| 3 | E | F | G | H | I | J | K | L |
��2�������й�Ԫ�����ʵĵݱ������ȷ����C
A����������ļ���E��F��G B����õ��ӵ�����I��J��K
C����̬�⻯����ȶ���D��K D��ԭ�Ӱ뾶H��I��J��K
��3�����ڵ������ڵķǽ���Ԫ�أ���Ar�⣩�������ң�ԭ�Ӱ뾶��С�����ʵ�����������ǿ������������Ӧˮ�������������ǿ��
 ��
�� ��1.76gHIO3������Ʒ�������ط������н������ط�������������ط������ߣ���Ʒ�������¶ȱ仯�����ߣ���ͼ��ʾ��
��1.76gHIO3������Ʒ�������ط������н������ط�������������ط������ߣ���Ʒ�������¶ȱ仯�����ߣ���ͼ��ʾ��