��Ŀ����
��Na2SO4��������0.2mol/L Na2SO4��Һ50mL��
��1����Ҫ�IJ���������50mL����ƿ���ձ�����Ͳ���������� ��
��2����������ƽ����Na2SO4���������Ϊ ��
��3�����в�����ʵ�����к�Ӱ�죨�ƫ�ߡ�����ƫ�͡�������Ӱ�족��
A�����ձ��е���Һע������ƿ��δϴ���ձ���
B������ʱ��������ƿ�Ŀ̶��ߣ�
C��ѡ�õ�����ƿ�ڲ�������������ˮ�� ��
��1����Ҫ�IJ���������50mL����ƿ���ձ�����Ͳ����������
��2����������ƽ����Na2SO4���������Ϊ
��3�����в�����ʵ�����к�Ӱ�죨�ƫ�ߡ�����ƫ�͡�������Ӱ�족��
A�����ձ��е���Һע������ƿ��δϴ���ձ���
B������ʱ��������ƿ�Ŀ̶��ߣ�
C��ѡ�õ�����ƿ�ڲ�������������ˮ��
���㣺����һ�����ʵ���Ũ�ȵ���Һ
ר�⣺���ʵ���Ũ�Ⱥ��ܽ��ר��
��������1������ʵ������IJ����Լ�ÿ��������Ҫ����ȷ����Ӧ����������
��2������m=nM=cvM���㣻
��3������c=
�������������ʵ����ʵ��������Һ�������Ӱ���жϣ�
��2������m=nM=cvM���㣻
��3������c=
| n |
| V |
���
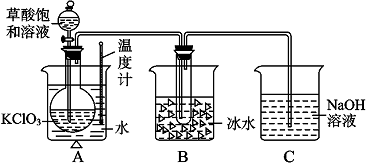
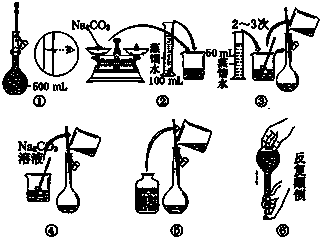
�⣺��1�����������г������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣬��ȴ��ת�Ƶ�500mL����ƿ�У����ò���������������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����Ҫ������Ϊ��ͷ�ιܣ��ʴ�Ϊ����ͷ�ιܣ�
��2��ʵ��������50mL 0.2mol/L��Na2SO4��Һ��ҪNa2SO4������Ϊ��0.05L��0.2mol/L��142g/mol=1.42g��������ƽ��ȷ��0.1��Ӧ����1.4 g���ʴ�Ϊ��1.4��
��3��A�����ù����ձ�δϴ�ӣ��������ʵ����ʵ���ƫС�����Ƶ���ҺŨ��ƫ�ͣ��ʴ�Ϊ��ƫ�ͣ�
B������ʱ������Һ��Һ���棬������Һ�����ƫС������������Һ��Ũ��ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
C������ƿ������ϴ�Ӻ������������ˮ����Ӱ�����ʵ����ʵ�������Һ�����������������Һ�����ʵ���Ũ����Ӱ�죬�ʴ�Ϊ����Ӱ�죮
��2��ʵ��������50mL 0.2mol/L��Na2SO4��Һ��ҪNa2SO4������Ϊ��0.05L��0.2mol/L��142g/mol=1.42g��������ƽ��ȷ��0.1��Ӧ����1.4 g���ʴ�Ϊ��1.4��
��3��A�����ù����ձ�δϴ�ӣ��������ʵ����ʵ���ƫС�����Ƶ���ҺŨ��ƫ�ͣ��ʴ�Ϊ��ƫ�ͣ�
B������ʱ������Һ��Һ���棬������Һ�����ƫС������������Һ��Ũ��ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
C������ƿ������ϴ�Ӻ������������ˮ����Ӱ�����ʵ����ʵ�������Һ�����������������Һ�����ʵ���Ũ����Ӱ�죬�ʴ�Ϊ����Ӱ�죮
���������⿼����һ�����ʵ���Ũ����Һ�����ƣ��ѶȲ��������ȡŨ��������ѡ����Ͳ��

��ϰ��ϵ�д�
 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�
�����Ŀ
 ��1����ijҩƷ����ԼΪ32g
��1����ijҩƷ����ԼΪ32g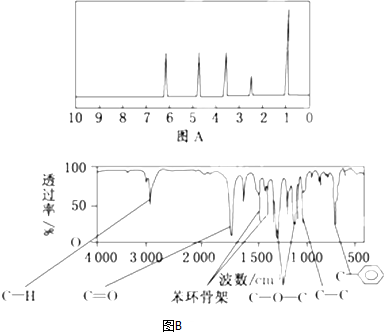
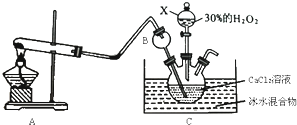
 ��1�����ʯ��ʯī��Ϊ̼��ͬ�������壬��������������ʱȼ������һ����̼������������ʱ���ȼ�����ɶ�����̼����Ӧ�зų���������ͼ��ʾ��
��1�����ʯ��ʯī��Ϊ̼��ͬ�������壬��������������ʱȼ������һ����̼������������ʱ���ȼ�����ɶ�����̼����Ӧ�зų���������ͼ��ʾ��