��Ŀ����
16�� Na2S2O3�׳ƴ��մ���������Ҫ�Ļ���ԭ�ϣ���Na2SO3�������ˮ��Һ�м��ȷ�Ӧ�������Ƶ�Na2S2O3����֪10���70��ʱ��Na2S2O3��ˮ�е��ܽ�ȷֱ�Ϊ60.0g��212g�������£�����Һ�������ľ�����Na2S2O3•5H2O��
Na2S2O3�׳ƴ��մ���������Ҫ�Ļ���ԭ�ϣ���Na2SO3�������ˮ��Һ�м��ȷ�Ӧ�������Ƶ�Na2S2O3����֪10���70��ʱ��Na2S2O3��ˮ�е��ܽ�ȷֱ�Ϊ60.0g��212g�������£�����Һ�������ľ�����Na2S2O3•5H2O����ʵ��������ȡNa2S2O3•5H2O���壨Na2S2O3•5H2O�ķ�����Ϊ248��
�������£�
�ٳ�ȡ12.6g Na2SO3���ձ��У�����80.0mLˮ��
����ȡ4.0g��ۣ��������Ҵ���ʪ�ӵ�������Һ�У�
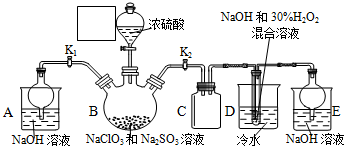
�ۣ���ͼ��ʾ������װ����ȥ����ˮԡ���ȣ��У���ӦԼ1Сʱ����ˣ�
����Һ�ھ�����������Na2S2O3•5H2O���壮
�ݽ��м�ѹ���˲����
��1������B�����������������ܣ������������������������������Ҵ���ʪ��Ŀ�������ӷ�Ӧ��Ӵ��������߷�Ӧ���ʣ�
��2�������Ӧ��ȡ�IJ���������Ũ������ȴ�ᾧ��
��3����Һ�г�Na2S2O3�Ϳ���δ��Ӧ��ȫ��Na2SO3�⣬����ܴ��ڵ���������Na2SO4��
��4��Ϊ�˲��Ʒ�Ĵ��ȣ���ȡ7.40g��Ʒ�����Ƴ�250mL��Һ������Һ����ȡ25.00mL����ƿ�У��μӵ�����Һ��ָʾ��������Ũ��Ϊ0.0500mol/L �ĵ�ˮ������ʽ�ζ������ζ���2S2O32-+I2=S4O62-+2I-�����ζ�������£�
| �ζ����� | �ζ�ǰ������mL�� | �ζ��ζ��������mL�� |
| ��һ�� | 0.00 | 30.82 |
| �ڶ��� | 0.00 | 30.80 |
| ������ | 0.00 | 30.78 |
���� ��1������װ��ͼ��֪������BΪ���������ܣ�����ƿ�е�Һ������������������������ˮ�������Ҵ����Ҵ�ʪ�����ʹ������ڷ�ɢ����Һ�У����ھƾ����ܣ���������Ӵ��������߷�Ӧ���ʣ�
��2����Һ�еõ����ʹ���ķ�����ͨ������Ũ������ȴ�ᾧ������ϴ�ӣ�����Ȳ���õ���
��3������S2O32?���л�ԭ�ԣ��ױ�������������������ӿ�֪����Ϊ�����ƣ�������������Ӽ��鷽���ʵ����飻
��4����ˮ�������ԣ��ܸ�ʴ�ܣ����ݻ�ѧ��Ӧ����ʽ2S2O32-+I2�TS4O42-+2I-���㼴�ɣ��ⶨ��Ʒ�Ĵ��Ⱦ����Ե����ҺΪ���ģ�����ζ��յ�û�п��ƺã������Һ�μӹ���Ҳ����������Ʒ�Ӧ��
��� �⣺��1������װ��ͼ��֪������BΪ���������ܣ�����ƿ�е�Һ������������������������ˮ�����Ҵ�����������ڷ�Ӧǰ���Ҵ�ʪ����ʹ������ڷ�ɢ����Һ�У���������ۺ�Na2SO3��Һ��ֽӴ����ӿ췴Ӧ����
�ʴ�Ϊ�����������ܣ��������������ӷ�Ӧ��Ӵ��������߷�Ӧ���ʣ�
��2��ͨ������Ũ������ȴ�ᾧ������ϴ�ӣ�����Ȳ���õ���Һ�е����ʹ��壬
�ʴ�Ϊ������Ũ������ȴ�ᾧ��
��3��S2O32?���л�ԭ�ԣ��ܹ���������������������ӣ���Һ�г�Na2S2O3�Ϳ���δ��Ӧ��ȫ��Na2SO3�⣬���ڱ��������������ƣ����Կ��ܴ��ڵ������������ƣ�
�ʴ�Ϊ��Na2SO4��
��4����ˮ�������ԣ��ܸ�ʴ�ܣ����Ե�ˮӦ������ʽ�ζ����н��еζ����������б��е����ݿ�֪���ڶ�������ƫ��ϴ�����ȡһ��������ʵ������ݣ�������ȥ�ĵ�ˮ�����Ϊ$\frac{30.78+30.82}{2}$mL=30.8mL��������ʵ���Ϊ��0.0500mol•L-l��0.0308L=0.00154mol��
2S2O32-+I2�TS4O62-+2I-��
2 1
x 0.00154mol
��x=0.00308mol����Na2S2O3•5H2O�����ʵ���Ϊ0.00308mol������Ϊ��0.00308��248g/mol=0.7638g��
���ȡ7.40g��Ʒ�����Ƴ�250mL��Һ�У�Na2S2O3•5H2O������Ϊ=0.7638g��$\frac{250}{25}$=7.638g
�ʲ�Ʒ�Ĵ���Ϊ��$\frac{7.638g}{7.40g}$��100%=103.2%��
�ⵥ����ǿ�������ԣ�Na2SO3���л�ԭ�ԣ�Na2SO3���I2������Ӧ���Ӷ�Ӱ�촿�ȣ�
�ʴ�Ϊ��103.2%�����е�Na2SO3Ҳ���I2������Ӧ���Ӷ�Ӱ�촿�ȣ�
���� ���⿼�����ʵ��Ʊ���Ϊ��Ƶ���㣬������ѧ���ķ�����ʵ��ͼ��������Ŀ��飬��Ŀ�漰�Լ������á����ʵ��ƶϡ��ζ��ļ��㡢����ʽ����д��֪ʶ���ζ�ʵ������ݴ����ͼ��㷽���ǽ���ؼ�����Ŀ�ѶȽϴ�ע���ϵʽ��Ӧ�ã�

 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�| A�� | 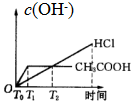 | B�� | 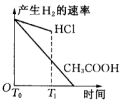 | C�� |  | D�� | 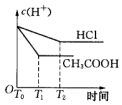 |
| �Ҵ� | ������ | ������ | 1-�嶡�� | |
| �ܶ�/��g•cm-3�� | 0.789 3 | 1.460 4 | 0.809 8 | 1.275 8 |
| �е�/�� | 78.5 | 38.4 | 117.2 | 101.6 |
��2����1-�嶡��ֲ�Ʒ���ڷ�Һ©���У���ˮ���ã��������²㣨��ϲ㡱���²㡱���ֲ㡱����
��3���Ʊ������У������Ũ����������ϡ�ͣ���Ŀ����ABC������ĸ����
A�����ٸ�����ϩ���ѵ����ɡ��� B������Br2������
C������HBr�Ļӷ� D��ˮ�Ƿ�Ӧ�Ĵ���
��4������ȥ������е���������Br2���������������ʺϵ���C������ĸ����
A��NaI B��NaOH C��NaHSO3 D��KCl
��5�����Ʊ�������ʱ�����ñ߷�Ӧ���������ķ�����Ŀ���ǣ�ƽ��������������ķ����ƶ�����Ӧ�������ƶ���
�����Ʊ�1-�嶡��ʱȴ���ܱ߷�Ӧ��������ԭ��1-�嶡����������ķе�����
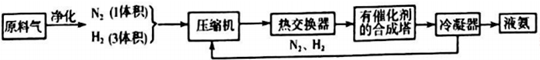
�ش��������⣺
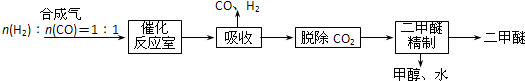
��1���ϳɰ�����ĵ�����Դ�ڿ������ϳɰ������ԭ��������������Ȼ���Ʊ�������Ҫ��ӦΪCH4��g��+H2O��g��=CO��g��+3H2��g����CH4��O2�ķ�Ӧ��2CH4��g��+O2��g��=2CO��g��+4H2��g����CH4��H2O��g��������������O2�����ϸߣ���ͬ��������������������ͬ����Ϸ�Ӧ������������������
| ���� | CO | H2 | N2 | O2 |
| �����L�� | 25 | 60 | 15 | 2.5 |
��2���ںϳɰ���ԭ�����л��е����ʱ����ȥ��ԭ���Ƿ�ֹ�����ж���
�����������Ƚ��������������Ƚ����������úϳɰ��Ͱ������ų�������������ԭ�������Ԥ�ȷ�Ӧ��������Ӻϳ��������Ļ�����壬ͨ��������15%������������İ���Ϊ���ԭ�ϵ������ʣ�ͨ����ȡ�Ĵ�ʩ�ǽ�N2��H2ѭ�����ã�
��3���ϳɵ�Ϊ���ȷ�Ӧ������ҵ�ϲ���400��-500����¶ȣ���Ҫԭ���ǣ�
�ٸ��¶ȷ�Χ�ڷ�Ӧ���ʽϿ죮�ڸ��¶��´����Ļ��Խϴ�
��4��������CO2��Ӧ�Ʊ����أ�CO��NH2��2]����Ӧ���̷�Ϊ��������д���йصĻ�ѧ����ʽ��
�ٵ����������̼�ڼ��ȼ�ѹ�����»������ɰ�������泥�H2NCOONH4����2NH3+CO2$\stackrel{���ȼ�ѹ}{��}$H2NCOONH4��
�ڰ�����������ȷֽ�Ϊ������ˮ��H2NCOONH4$\stackrel{��}{��}$CO��NH2��2+H2O��
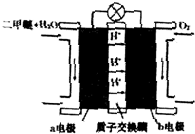
��5���·��ϳɰ������õ�ⷨ�ϳɣ�����ѹ�½�������������ϡ�͵ĵ����ֱ�ͨ��һ�����ȵ�570��ĵ����У������͵����ڵ缫�Ϻϳ��˰����������˰��IJ��ʣ��·��ϳɰ����õĵ�����ܴ���H+���������ĵ缫��ӦʽΪN2+6H++6e-�T2NH3��
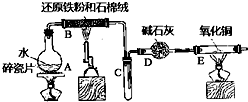 ijʵ��С��������ͼ����װ�ý��С�����ˮ������Ӧ����ʵ�飬�����ò����һ����ȡFeCl3•6H2O���壮��ͼ�мгּ�β������װ�þ�����ȥ����ش��������⣺
ijʵ��С��������ͼ����װ�ý��С�����ˮ������Ӧ����ʵ�飬�����ò����һ����ȡFeCl3•6H2O���壮��ͼ�мгּ�β������װ�þ�����ȥ����ش��������⣺