��Ŀ����
5���ɼ������ӻ�������ɵĻ����������������е������֣�Na+��Cl-��NH4+��Ca2+��Cu2+��CO32-��Ba2+��SO42-�����û��������ˮ�����ɫ������Һ����ȡ3��100mL����Һ�ֱ��������ʵ�飺| ʵ����� | ʵ������ | ʵ���� |
| 1 | ��AgNO3��Һ | �а�ɫ�������� |
| 2 | ������NaOH��Һ������ | �ռ�������2.24L��������ɱ�״���� |
| 3 | ������BaCl2��Һʱ�������ó�������ϴ�ӡ������������������м�����ϡ���ᣬȻ�������� | ��һ�γ�������Ϊ6.27g���ڶ��γ�������Ϊ2.33g |
��1������ʵ��1��3�жϻ������һ�������ڵ�������Cu2+��Ba2+��Ca2+��
��2����ȷ����Һ��һ�����ڵ������Ӽ������ʵ���Ũ�ȣ��ɲ���������
| �����ӷ��� | ���ʵ���Ũ�ȣ�mol/L�� |
���� 1������AgNO3��Һ�г���������˵����Һ�п��ܴ���Cl-��CO32-��SO42-��
2.2.24LΪ���������������ʵ���Ϊ0.1mol����Һ��һ������NH4+���������ʵ���Ϊ0.1mol��
3.2.33gΪ���ᱵ�����ᱵ�����ʵ���Ϊ0.01mol��6.27gΪ���ᱵ��̼�ᱵ��̼�ᱵ������Ϊ��6.27g-2.33g=3.94g�����ʵ���Ϊ0.02mol����Һ��һ����SO42-��CO32-���������ӹ����֪��һ��������Cu2+��Ba2+��Ca2+����ϵ���غ�����⣮
��� �⣺1������AgNO3��Һ�г���������˵����Һ�п��ܴ���Cl-��CO32-��SO42-��
2.2.24LΪ���������������ʵ���Ϊ0.1mol����Һ��һ������NH4+���������ʵ���Ϊ0.1mol��
3.2.33gΪ���ᱵ�����ᱵ�����ʵ���Ϊ0.01mol��6.27gΪ���ᱵ��̼�ᱵ��̼�ᱵ������Ϊ��6.27g-2.33g=3.94g�����ʵ���Ϊ0.02mol����Һ��һ����SO42-��CO32-���������ӹ����֪��һ��������Cu2+��Ba2+��Ca2+��
��1�������Ϸ�����֪��һ��������Cu2+��Ba2+��Ca2+���ʴ�Ϊ��Cu2+��Ba2+��Ca2+��
��2����ϣ�1���з�����֪��Һ��һ�����е�������ΪCO32-��SO42-����̼�ᱵ���������ᣬ���ᱵ�������������֪���������ʣ��2.33g����ΪBaSO4���������غ��֪��Һ��c��SO42-��=$\frac{\frac{2.33g}{233g/mol}}{0.1L}$=0.1mol/L��6.27g������̼�ᱵ������Ϊ6.27g-2.33g=3.94g������̼�غ��֪��Һ��c��CO32-��=$\frac{\frac{3.94g}{197g/mol}}{0.1L}$=0.2mol/L���ʴ�Ϊ��
| �����ӷ��� | ���ʵ���Ũ�ȣ�mol/L�� |
| SO42- | 0.1 |
| CO32- | 0.2 |
�ʴ�Ϊ��һ�����ڣ�ȡ������Һ���Թ��У�����������Ba��NO3��2��������ȫ����ȡ�ϲ���Һ���μ�HNO3�ữ��AgNO3���а�ɫ����������˵����Cl-
���� ���⿼���˳������ӵļ��鷽����Ϊ��Ƶ���㣬��Ŀ�ѶȽϴ������ϴ������漰�˳������ӵļ��鷽���жϣ����и��ݵ���غ��ж������ӵĴ���Ϊ�ѵ���״��㣮

| A�� | �ƹ�ʹ�ú���ϴ�Ӽ� | B�� | �ù�ҵ��ˮֱ�ӹ��ũ�� | ||
| C�� | ��O3���Cl2������ˮ������ | D�� | ��Hg2+�ķ�ˮֱ���ŷ� |
| A�� | SO2��ˮ��Һ���Ե���˵����SO2����� | |
| B�� | ����NaOH��Һʱ����ʹ�ò����Թܣ���Ϊ�����е�SiO2����NaOH��Ӧ | |
| C�� | ij��ɫ��Һ�м�������������Һ�����ȣ�������������ʹʪ���ɫʯ����ֽ����������Һһ����NH4+ | |
| D�� | ij��Һ�м��������ܲ���ʹ����ʯ��ˮ����ǵ����壬�����Һ��һ������CO32-��HCO3- |
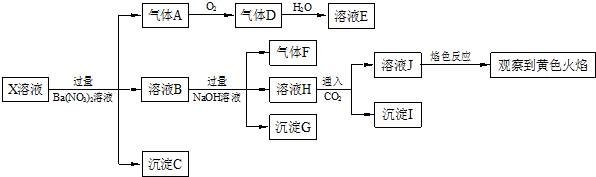
���н�����ȷ���ǣ�������
| A�� | X�п϶�����Na+��Fe2+��A13+��NH4+��SO42- | |
| B�� | ����F����������ֱ����������D | |
| C�� | ����Cһ����BaSO4������Gһ����Fe��OH��3������Iһ����Al��OH ��3 | |
| D�� | X�в���ȷ���������� A13+��Na+��K+��C1- |
| A�� | ǿ���ǿ����Һ�ķ�Ӧ������Ƴ�ԭ��� | |
| B�� | ��������п��������ʱ��������Һ�Ӵ���п����ʴ | |
| C�� | �����ڷ����ķ�Ӧ��Ϊ�Է����е�������ԭ��Ӧ | |
| D�� | �ö��Ե缫�����������ʳ��ˮ�ɼ�����ʹ��Һ�ָ���ԭ״̬ |
| A�� | Ħ����һ�ֹ��ʻ��������� | |
| B�� | 1 mol�������Ϊ1g | |
| C�� | ��ͬ��ͬѹ�£���ͬ������κ����嵥��������������ͬ | |
| D�� | ��״��������Ħ�����ԼΪ22.4 L |
| A�� | ̼����������Һ��Ӧ��CaCO3+2H +�TCa2++CO2��+H2O | |
| B�� | ����ʯ��ˮ�����ᷴӦ��OH-+H+�TH2O | |
| C�� | ��Na2O2����ˮ��Na2O2+H2O�T2Na++2OH-+O2�� | |
| D�� | ƫ��������Һͨ�����CO2��AlO2-+4CO2+2 H2O�TAl3++4 HCO3- |
��ʾ����H2C2O4�Ƕ�Ԫ����
��10[KHC2O4•H2C2O4]+8KMnO4+17H2SO4�T8MnSO4+9K2SO4+40CO2��+32H2O��
| A�� | 0.0800 | B�� | 0.1200 | C�� | 0.1600 | D�� | 0.2400 |
