��Ŀ����
��1��ijȲ��B��H2��ּӳɺ�����2��5-�������飬��B�Ľṹ��ʽΪ ��
��2��̼ԭ������10���ڵ�����������ͬ���칹���У�һ�ȴ�����ͬ���칹����� ��
��3��ijһԪ����A����̼����������Ϊ50.0%���������塢�廯�ⶼ���Ը�A��ӳɷ�Ӧ������A�Ľṹ��ʽ
��4����һ����������ƿ��ʢ10g11.6%��ijȩ��Һ��Ȼ����������������Һ��ֻ�Ϸ�����ˮԡ�м��ȣ���ȫ��Ӧ��ȥƿ��Һ�壬��ϸϴ������ɺ���ƿ��������4.32g��ͨ�����㣬д������ȩ�Ľṹ��ʽ
��5��0.2molij�л����0.4mol O2���ܱ�������ȼ�պ���ΪCO2��CO��H2O�����ᆳ��ŨH2SO4��ŨH2SO4����10.8g��ͨ�����ȵ�CuO��ַ�Ӧ��CuOʧ��3.2g�����ͨ����ʯ�ң���ʯ������17.6g�����������л�����9.2g ��ǡ�÷�Ӧ�����㲢�ش� ���л�������� ��
��2��̼ԭ������10���ڵ�����������ͬ���칹���У�һ�ȴ�����ͬ���칹�����
��3��ijһԪ����A����̼����������Ϊ50.0%���������塢�廯�ⶼ���Ը�A��ӳɷ�Ӧ������A�Ľṹ��ʽ
��4����һ����������ƿ��ʢ10g11.6%��ijȩ��Һ��Ȼ����������������Һ��ֻ�Ϸ�����ˮԡ�м��ȣ���ȫ��Ӧ��ȥƿ��Һ�壬��ϸϴ������ɺ���ƿ��������4.32g��ͨ�����㣬д������ȩ�Ľṹ��ʽ
��5��0.2molij�л����0.4mol O2���ܱ�������ȼ�պ���ΪCO2��CO��H2O�����ᆳ��ŨH2SO4��ŨH2SO4����10.8g��ͨ�����ȵ�CuO��ַ�Ӧ��CuOʧ��3.2g�����ͨ����ʯ�ң���ʯ������17.6g�����������л�����9.2g ��ǡ�÷�Ӧ�����㲢�ش� ���л��������
���㣺�й��л������ʽȷ���ļ���,�л���ʵ��ʽ�ͷ���ʽ��ȷ��,�л���������칹����
ר�⣺�������������ȼ�չ���
��������1����������2��5-���������̼�ܣ��ڸ�������̼��������̼̼�������ɣ�
��2��̼ԭ������10���ڵ�����������ͬ���칹���У�һ�ȴ�����ͬ���칹�壬˵����������ֻ��һ�ֵ�Ч��ԭ�ӣ����ݵ�Ч��ԭ�ӵ��жϷ������ش�
��3���������塢�廯�ⶼ���Ը�A��ӳɷ�Ӧ��˵��A�к��в����ͼ���A��һԪ���ᣬ����A����-COOH�����ݺ�̼����������Ϊ50.0%��⣻
��4����ƿ��������4.32g��ΪAg�������ʵ���n=
=0.04mol������R-CHO��2Ag�����۽�𣻣�5��Ũ���������ˮ�ԣ�������������10.8g��˵����Ӧ�����к�ˮ10.8g��ͨ����������ͭ�����ڷ�����ӦCuO+CO
Cu+CO2����������������3.2g����Ϸ���ʽ���ò������ɼ���CO�����ʵ�����ͨ����ʯ��ʱ����ʯ�ҵ�����������17.6g�ɼ�����CO2�����ʵ�������ȥCO��CuO��Ӧ���ɵ�CO2������Ϊ�л���ȼ������CO2������������Ԫ���غ�����л����к���C��H��O�����ʵ�����������û�ѧʽ����Ϸ���ʽ�����Ʒ�Ӧ�����ʵ���ȷ�����������༰��Ŀ���ݴ���д�л�������ƣ�
��2��̼ԭ������10���ڵ�����������ͬ���칹���У�һ�ȴ�����ͬ���칹�壬˵����������ֻ��һ�ֵ�Ч��ԭ�ӣ����ݵ�Ч��ԭ�ӵ��жϷ������ش�
��3���������塢�廯�ⶼ���Ը�A��ӳɷ�Ӧ��˵��A�к��в����ͼ���A��һԪ���ᣬ����A����-COOH�����ݺ�̼����������Ϊ50.0%��⣻
��4����ƿ��������4.32g��ΪAg�������ʵ���n=
| 4.32g |
| 108g/mol |
| ||
���
�⣺��1��2��5-���������̼��Ϊ��C-C��C��-C-C-C��C��-C���ڸ�̼��������̼̼���������Եõ�Ȳ������Ȳ���Ľṹ��ʽΪ��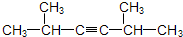 ������CH3��2CHC��CCH��CH3��2��
������CH3��2CHC��CCH��CH3��2��
�ʴ�Ϊ����CH3��2CHC��CCH��CH3��2��
��2�����������У�ͬһ��̼�ϵ���ԭ�ӵ�Ч������ͬһ��̼ԭ���ϵ���ԭ�ӵ�Ч�����о���ԳƵ�̼ԭ���ϵ���ԭ�ӵ�Ч��������һ��ȡ����ֻ��һ�֣�˵����������ֻ��һ�ֵ�Ч��ԭ�ӣ���̼ԭ����n��10������������ͬ���칹���У���һ��ȡ����ֻ��һ�ֵ������ֱ��ǣ����顢���顢2��2-���������Լ�2��2��3��3-�ļ����飬
�ʴ�Ϊ��4��
��3�����һԪ�������ʽΪCnHmO2���ɺ�̼����������Ϊ50%���ã�12n=m+32���������塢�廯�ⶼ���Ը�A��ӳɷ�Ӧ��˵��A�к��в����ͼ������ۿɵã�n=3��m=4����C3H4O2��Ϊ��ϩ�ᣬ��ṹ��ʽΪ��CH2=CH-COOH��
�ʴ�Ϊ��CH2=CHCOOH��
��4����ƿ��������4.32g��ΪAg�������ʵ���n=
=0.04mol��10g11.6%��ijȩ��Һȩ�����ʵ���Ϊ10��11.6%=1.16g��
��ΪһԪȩ����һԪȩ�����ʵ���Ϊx��
�� R-CHO��2Ag
1mol 2mol
x 0.04mol x=0.02mol��
RCHO��Ħ������M=
=
=58g/mol������ȩͨʽCnH2nO��Ϊ��ȩCH3CH2CHO��
��Ϊ��Ԫȩ ����
OHC-RCHO��4Ag
1mol 4mol
0.01mol 0.04mol
���Ԫȩ��Ħ������M=
=
=116g/mol��-CHOΪ29g/mol����R=116-29��2=58����������ͬʱ���ų���ȩ�Ŀ����ԣ�
�ʴ�Ϊ��CH3CH2CHO��
��5���л���ȼ������ˮ10.8g��ˮ�����ʵ���Ϊ��
=0.6mol��
���л���ȼ�����ɵ�CO����Ϊx��
��CuO+CO
Cu+CO2����̬���ء�m
28g 16g
x 3.2g
����x=
=5.6g��CO�����ʵ���Ϊ��
=0.2mol��
����̼Ԫ���غ��֪CO��CuO��Ӧ���ɵ�CO2�����ʵ���Ϊ0.2mol������Ϊ��0.2mol��44g/mol=8.8g��
�л���ȼ�����ɵ�CO2������Ϊ��17.6g-8.8g=8.8g�����ʵ���Ϊ��
=0.2mol��
����̼Ԫ���غ��֪��1mol�л��ﺬ��̼ԭ�����ʵ���Ϊ��
mol=2mol��
������Ԫ���غ��֪��1mol�л��ﺬ����ԭ�����ʵ���Ϊ��
mol=6mol
������Ԫ���غ��֪��1mol�л��ﺬ����ԭ�����ʵ���Ϊ��
=2mol��
�����л���ķ���ʽΪ��C2H6O2��9.2g ��Ϊ0.4mol��0.2mol���л�����0.4mol��ǡ�÷�Ӧ���л����к���2���ǻ������л���Ľṹ��ʽΪOHCH2CH2OH�����л��������Ϊ�Ҷ�����
�ʴ�Ϊ���Ҷ�����
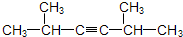 ������CH3��2CHC��CCH��CH3��2��
������CH3��2CHC��CCH��CH3��2���ʴ�Ϊ����CH3��2CHC��CCH��CH3��2��
��2�����������У�ͬһ��̼�ϵ���ԭ�ӵ�Ч������ͬһ��̼ԭ���ϵ���ԭ�ӵ�Ч�����о���ԳƵ�̼ԭ���ϵ���ԭ�ӵ�Ч��������һ��ȡ����ֻ��һ�֣�˵����������ֻ��һ�ֵ�Ч��ԭ�ӣ���̼ԭ����n��10������������ͬ���칹���У���һ��ȡ����ֻ��һ�ֵ������ֱ��ǣ����顢���顢2��2-���������Լ�2��2��3��3-�ļ����飬
�ʴ�Ϊ��4��
��3�����һԪ�������ʽΪCnHmO2���ɺ�̼����������Ϊ50%���ã�12n=m+32���������塢�廯�ⶼ���Ը�A��ӳɷ�Ӧ��˵��A�к��в����ͼ������ۿɵã�n=3��m=4����C3H4O2��Ϊ��ϩ�ᣬ��ṹ��ʽΪ��CH2=CH-COOH��
�ʴ�Ϊ��CH2=CHCOOH��
��4����ƿ��������4.32g��ΪAg�������ʵ���n=
| 4.32g |
| 108g/mol |
��ΪһԪȩ����һԪȩ�����ʵ���Ϊx��
�� R-CHO��2Ag
1mol 2mol
x 0.04mol x=0.02mol��
RCHO��Ħ������M=
| m |
| n |
| 1.16g |
| 0.02mol |
��Ϊ��Ԫȩ ����
OHC-RCHO��4Ag
1mol 4mol
0.01mol 0.04mol
���Ԫȩ��Ħ������M=
| m |
| n |
| 1.16g |
| 0.01mol |
�ʴ�Ϊ��CH3CH2CHO��
��5���л���ȼ������ˮ10.8g��ˮ�����ʵ���Ϊ��
| 10.8g |
| 18g/mol |
���л���ȼ�����ɵ�CO����Ϊx��
��CuO+CO
| ||
28g 16g
x 3.2g
����x=
| 28g��3.2g |
| 16g |
| 5.6g |
| 28g/mol |
����̼Ԫ���غ��֪CO��CuO��Ӧ���ɵ�CO2�����ʵ���Ϊ0.2mol������Ϊ��0.2mol��44g/mol=8.8g��
�л���ȼ�����ɵ�CO2������Ϊ��17.6g-8.8g=8.8g�����ʵ���Ϊ��
| 8.8g |
| 44g/mol |
����̼Ԫ���غ��֪��1mol�л��ﺬ��̼ԭ�����ʵ���Ϊ��
| 0.2mol+0.2mol |
| 0.2mol |
������Ԫ���غ��֪��1mol�л��ﺬ����ԭ�����ʵ���Ϊ��
| 0.6mol��2 |
| 0.2 |
������Ԫ���غ��֪��1mol�л��ﺬ����ԭ�����ʵ���Ϊ��
| 0.6mol+0.2mol+0.2mol |
| 0.2 |
�����л���ķ���ʽΪ��C2H6O2��9.2g ��Ϊ0.4mol��0.2mol���л�����0.4mol��ǡ�÷�Ӧ���л����к���2���ǻ������л���Ľṹ��ʽΪOHCH2CH2OH�����л��������Ϊ�Ҷ�����
�ʴ�Ϊ���Ҷ�����
���������⿼���˳����л������ʽ���ṹ��ʽ��ȷ���ļ��㣬���ؿ���ѧ���������������������Ŀ�Ѷ��еȣ�ע������ȷ���л������ʽ���ṹʽ�ķ������ܹ����ݷ�Ӧ�����ж��л�������к��еĹ��������ͼ���Ŀ�ǽ����Ĺؼ���ע�����ü��跨���н��4����ע�������ȩ��������Һ��Ӧ��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
�ñ�������������Һ��ָʾ��Ϊ��̪���ζ�ijδ֪Ũ�ȵ����ᣬ���²�����ɲⶨ���ƫ�͵��ǣ�������
| A�����Ʊ���Һ���õ����������Ѿ����� |
| B���ζ��յ����ʱ�����ӵζ��ܵĿ̶ȣ�������������ȷ |
| C��δ�ñ�Һ��ϴ��ʽ�ζ��� |
| D��ʢװδ֪Һ����ƿ������ˮϴ����δ�ô���Һ��ϴ |
ʵ���������� 100mL 1.5mol/L NaOH ��Һ������������ȷ���ǣ�������
| A��ת��Һ��ʱ��������Ӧ��������ƿ�� |
| B������ƿ�ϱ����¶ȡ�Ũ�ȡ��ݻ���ѹǿ |
| C���������6.0g�������ƹ��嵹�뵽����ƿ���ܽ� |
| D������ʱ�����ӿ̶��߹۲�Һ�棬��ʹ�����Ƶ�NaOH ��Һ��Ũ��ƫ�� |
һԪ��HA��Һ�У�����һ����ǿ��MOH��Һ��ǡ����ȫ��Ӧ����Ӧ�����Һ�У������ж�һ����ȷ���ǣ�������
| A��c��A-���Rc��M+�� |
| B��c��A-��=c��M+�� |
| C����MA��ˮ�⣬��c��OH-����c��H+�� |
| D����MAˮ�⣬��c��OH-����c��H+�� |
��֪ij�¶���0.1moL/L��NaHB��Һ��c��H+����c��OH-���������й�ϵʽһ����ȷ���ǣ�������
| A��c��H+��+c��Na+��=c��HB-��+c��B2-��+c��OH-�� |
| B��c��Na+��=0.1 moL/L��c��B2-�� |
| C��c��H+����c��OH-��=1��10-14 |
| D����Һ��pH=1 |
NaCl��Һ�л���Na2S��NaI��Ϊ�˳�ȥ���ʣ�����������һ���룬ѡ�����²�����
��ͨ��Cl2���ڼӵ�ˮ������CCl4��ȡ���ܹ��ˣ��ݷ�Һ�������˳����ȷ���ǣ�������
��ͨ��Cl2���ڼӵ�ˮ������CCl4��ȡ���ܹ��ˣ��ݷ�Һ�������˳����ȷ���ǣ�������
| A���٢ڢۢܢ� |
| B���ڢܢ٢ۢ� |
| C���ܢݢۢڢ� |
| D���ݢڢ٢ܢ� |