��Ŀ����
17��ʵ���У�ijͬѧ��������ƿ���ʵ���Ũ����ȵ�HCl��Һ��CH3COOH��Һ��װƿ����������ǩ��Ϊ����������ƿ�Լ�����������ѧ֪ʶ��������ַ�������1������һ�������Լ�ⷨ��ʵ����С���ݽ����ĵ�����Һ��HCl��Һ��
��2����������pH��ⷨ���÷����ľ������������ȡ����pH��ֽ���ڱ������ϣ��ýྻ�������ֱ�պȡ����Һ�㵽��ֽ���м䣬Ȼ�����ɫ���Ƚϣ�ȷ����pH��pH�������CH3COOH��Һ��
��3����������ͨ����ѧ��Ӧ�۲�������������������Һ�зֱ�Ͷ����ͬ���Ⱥ���״��п������ʼ����H2�����ʣ�HCl��Һ��CH3COOH��Һ�����������������=�������ղ���H2�������HCl��Һ=CH3COOH��Һ��
���� ��1����Һ������Ũ��Խ����Һ�ĵ�����Խǿ��
��2��ȡ����pH��ֽ���ڱ������ϣ��ⶨ������Һ��pH�������pH��
��3��Ũ����ȵ�HCl��Һ��CH3COOH��Һ��������������Ũ��С����Zn��Ӧ��������������С������ͬ��п��Ӧ���ɵ�������ͬ��
��� �⣺��1����Һ������Ũ��Խ����Һ�ĵ�����Խǿ��������ȫ���룬��Һ������Ũ�ȴ��ڴ���������Ũ�ȣ���������ĵ�����ǿ����ͨ�������С���ݽ�����
�ʴ�Ϊ��HCl��Һ��
��2��ȡ����pH��ֽ���ڱ������ϣ��ýྻ�������ֱ�պȡ����Һ�㵽��ֽ���м䣬��ɫ��Ȼ�����ɫ���Ƚϣ�ȷ����pH�����ڴ��Ჿ�ֵ��룬��Һ��������Ũ��С�����Դ����pH��
�ʴ�Ϊ��ȡ����pH��ֽ���ڱ������ϣ��ýྻ�������ֱ�պȡ����Һ�㵽��ֽ���м䣬Ȼ�����ɫ���Ƚϣ�ȷ����pH��CH3COOH��Һ��
��3��Ũ����ȵ�HCl��Һ��CH3COOH��Һ�����Ჿ�ֵ��룬������������Ũ��С����Zn��Ӧ��������������С�����ʵ���Ũ����ȵ�HCl��Һ��CH3COOH��Һ�������ͬʱ��������ʵ�����ͬ������п��Ӧ���ɵ�������ͬ��
�ʴ�Ϊ������=��
���� ���⿼����̽������ǿ����ʵ�鷽����ƣ���Ŀ�Ѷ��еȣ������ڿ���ѧ����ʵ��̽�������ͷ���������ע����մ���ĵ����ص㣮

 ��У����ϵ�д�
��У����ϵ�д�
| A�� | H2R�Ƕ�Ԫ���ᣬ��Kal=1��10-2 | |
| B�� | ����Һǡ�ó�����ʱ��c��Na+��=2c��R2-��+c��HR-�� | |
| C�� | NaHR����Һ��ˮ��������ڵ������� | |
| D�� | ��Na2R��NaHR��0.1mol�Ļ����Һ��pH=7.2 |
| A�� | C-H֮��ֻ��sp2�γɵĦҼ���C-C֮��ֻ��δ�μ��ӻ���2p����γɵĦм� | |
| B�� | C-C����sp2�γɵĦҼ���C-H֮����δ�μ��ӻ���2p����γɵĦм� | |
| C�� | sp2�ӻ�����γɦҼ���δ�ӻ���2p����γɦм� | |
| D�� | sp2�ӻ�����γɦм���δ�ӻ���2p����γɦҼ� |
| A�� | �����£�1 L pH=13��NaOH��Һ�У���ˮ�����OH-��ĿΪ0.1NA | |
| B�� | 2.0gH218O��D216O�Ļ����������������ΪNA | |
| C�� | 50ml 12mol/L����������MnO2���ȣ�ת�Ƶĵ�����Ϊ0.3NA | |
| D�� | ���³�ѹ�£�4.4g��ȩ�����Ҽ���ĿΪ0.7NA |
��Ca��OH��2
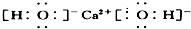
��H2S
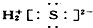
��OH-

��Al3+Al3+
��N2
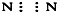
��CO2

��HClO
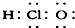
��Na2O2
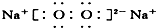
| A�� | �٢ڢۢ� | B�� | �ݢޢߢ� | C�� | �ڢۢݢޢ� | D�� | �٢ܢ� |
| A�� | R�ǵ�������Ԫ�� | |
| B�� | RԪ�ص����������NԪ�����������ͬ | |
| C�� | RO3-��NO3-��ֻ�ܱ���ԭ�����ܱ����� | |
| D�� | R��N��Ԫ�صĵ��ʶ��ǿ�������Ҫ�ɷ� |
| A�� | H��Cl | B�� | H[��O��]2- H | C�� | Mg2+ 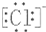 2 2 | D�� | Na+  |