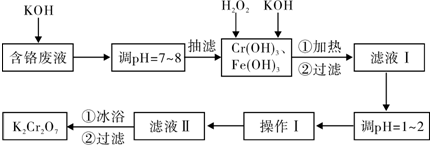
��Ŀ����
�йش����Ĵ�������������Դӡ��Ҵ�������ʵ�顱�õ�һЩ��ʶ��ij��ʦ�������ͼװ�ã��г�װ��������ʡ�ԣ�����ʵ�����Ϊ����ͼ��װ�ã��ȹرջ���a��b��c����ͭ˿���м䲿�ּ���Ƭ�̣�Ȼ�����a��b��c��ͨ�����ƻ���a��b�����н��ࣨ��Ъ�ԣ�ͨ�����壬������M���۲쵽���Ե�ʵ�������Իش��������⣺

��1��A�з�����Ӧ�Ļ�ѧ����ʽ�� ��C����ˮ�����ã� ��
��2��M���л�����뷴Ӧ�Ļ�ѧ����ʽΪ�� ��
��3������װ������ϵ�ȱ�ݣ�ʵ�����ʱ���ܻ ��

��1��A�з�����Ӧ�Ļ�ѧ����ʽ��
��2��M���л�����뷴Ӧ�Ļ�ѧ����ʽΪ��
��3������װ������ϵ�ȱ�ݣ�ʵ�����ʱ���ܻ
���㣺�Ҵ��Ĵ�����ʵ��
ר�⣺ʵ����
��������1��A���ǹ��������ڶ������̴�����������ˮ��������C�Ǽ����Ҵ��õ��Ҵ���������M��
��2��M�����Ҵ��Ĵ�������Ӧ������ȩ��
��3��������ֱ�Ӳ���ˮҺ���£�����������
��2��M�����Ҵ��Ĵ�������Ӧ������ȩ��
��3��������ֱ�Ӳ���ˮҺ���£�����������
���
�⣺�Ҵ�������ʵ�飺A���ǹ��������ڶ������̴�����������ˮ��������B�����������е�ˮ������C�Ǽ����Ҵ��õ��Ҵ���������M��M�������ķ�Ӧ���Ҵ��Ĵ�������Ӧ������ȩ��FΪ��ȩ�������Ҵ�����Һ��
��1��A���ǹ��������ڶ������̴�����������ˮ����������Ӧ�Ļ�ѧ����ʽΪ2H2O2
2H2O+O2����B�����������е�ˮ������C����ˮʹ�Ҵ���Ϊ��������M�вμӷ�Ӧ���ṩƽ��������
�ʴ�Ϊ��2H2O2
2H2O+O2����C����ˮʹ�Ҵ���Ϊ��������M�вμӷ�Ӧ���ṩƽ��������
��2��M�������ķ�Ӧ���Ҵ��Ĵ�������Ӧ������ȩ����Ӧ�Ļ�ѧ����ʽΪ��2CH3CH2OH+O2
2CH3CHO+2H2O��
�ʴ�Ϊ��2CH3CH2OH+O2
2CH3CHO+2H2O��
��3��������ֱ�Ӳ���ˮҺ���£����ɵ���ȩ�������Ҵ�������ˮ��ʹFװ�ò�����������
�ʴ�Ϊ������������
��1��A���ǹ��������ڶ������̴�����������ˮ����������Ӧ�Ļ�ѧ����ʽΪ2H2O2
| ||
�ʴ�Ϊ��2H2O2
| ||
��2��M�������ķ�Ӧ���Ҵ��Ĵ�������Ӧ������ȩ����Ӧ�Ļ�ѧ����ʽΪ��2CH3CH2OH+O2
| Cu |
| �� |
�ʴ�Ϊ��2CH3CH2OH+O2
| Cu |
| �� |
��3��������ֱ�Ӳ���ˮҺ���£����ɵ���ȩ�������Ҵ�������ˮ��ʹFװ�ò�����������
�ʴ�Ϊ������������
���������⿼�����������ڵ�ʵ����֤������ʵ����ƣ���Ҫ�ǹ�������ķֽⷴӦ���Ҵ��Ĵ����������жϺͼ��飬��Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
��������������ԭ�����͵��ǣ�������
| A���ȵĴ�����Һȥ����Ч���� |
| B���ϳɰ���ҵ��ʹ������ý������ |
| C��ʵ���ҳ����ű���ʳ��ˮ�ķ����ռ�Cl2 |
| D��ʵ����������FeCl3��Һʱ��Ӧ�����м�������Ũ���� |
һ��������ӦN2+3H2?2NH3��H��0��ƽ�⣬�������ı������������й�����������ǣ�������
| A���Ӵ�����V����V���������仯���ұ仯�ı������ |
| B������ѹǿ��V����V����������V�����ӱ�������V�����ӱ��� |
| C�������¶ȣ�V����V�������٣���V�����ٱ�������V�����ٱ��� |
| D��ͨ�������V����V����������V�����ӱ�������V�����ӱ��� |
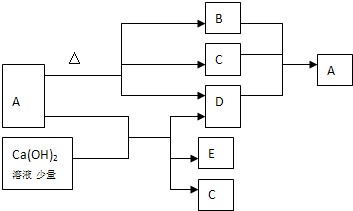
 A��B��C��D����ѧ��ѧ�ij������ʣ�����A��B��C������ͬһ��Ԫ�أ���һ���������ת���Ĺ�ϵ����ͼ��ʾ�����ַ�Ӧ�е�H2 O����ȥ��������գ�
A��B��C��D����ѧ��ѧ�ij������ʣ�����A��B��C������ͬһ��Ԫ�أ���һ���������ת���Ĺ�ϵ����ͼ��ʾ�����ַ�Ӧ�е�H2 O����ȥ��������գ�