��Ŀ����
17�����û�ѧԭ�����ԶԹ����ŷŵķ�ˮ�������Ƚ�����Ч���ʹ�����ij�������Ƹ���ҵ������Cr���Ļ����������ù������£������Һ�н���������Ҫ��Cr3+�������Fe3+��Fe2+��Al3+��Ca2+��Mg2+����
�����²��������ӵ����������γɳ���ʱ��Һ��pH���±���
| ������ | Fe3+ | Fe2+ | Mg2+ | Al3+ | Ca2+ | Cr3+ |
| ��ʼ����ʱ��pH | 1.9 | 7.0 | 9.6 | 4.2 | 9.7 | |
| ������ȫʱ��pH | 3.2 | 9.0 | 11.1 | 8.0 | 11.7 | 9.0����9.0 �ܽ⣩ |
��2����pH=4.0��Ϊ�˳�ȥFe3+
��3�������ӽ�����֬������ԭ��ΪMn++nNaR��MRn+nNa+��������������������Ca2+��Al3+��Mg2+
��4������ƽ����������ԭ��Ӧ����ʽ��2Fe2++1H2O2+2H+�T2Fe3++2H2O��������
��5��ͨ��SO2��Ŀ���ǽ����۸�Ԫ�ػ�ԭ�����۸�Ԫ�أ�����Cr��OH����H2O��5SO4��
���� ��1�����ʱ��Ϊ����߽�ȡ�ʿɲ�ȡ�Ĵ�ʩ���ӳ���ȡʱ�䡢�ӿ��ܽ��ٶȵȴ�ʩ�������ȡҺ�еĽ���������Ҫ��Cr3+�������Fe3+��Al3+��Ca2+��Mg2+��������ܽ�����Ϊ����߽�ȡ�ʣ����������¶����������ܽ�ȣ�����Ӵ��������Ӧ���ʣ���ӿ�����ٶȵȣ�
��2�������ȡҺ�еĽ���������Ҫ��Cr3+�������Fe3+��Al3+��Ca2+��Mg2+������NaOH��Һʹ��Һ����pH=4��Fe3+ת��Ϊ������ȥ��
��3�������ӽ�����֬�����������Ǹ����Ӻ�þ���ӡ������ӣ�
��4��˫��ˮ��ǿ�����ԣ��ܽ��������������������ӣ�����������ԭ��Ӧ�����غ㡢ԭ���غ���ƽ��д���ӷ���ʽ��
��5����������ͼ�е�ת����ϵ�Ͳ����϶�������Ļ�ԭ�Կ�֪�����������ܽ����۸�Ԫ�ػ�ԭ�����۸�Ԫ�أ�����Cr��OH����H2O��5SO4��
��� �⣺��1�����ʱ��Ϊ����߽�ȡ�ʿɲ�ȡ�Ĵ�ʩ�ǣ����ӽ�ȡʱ�䡢���Ͻ������������ν�ȡ�ȣ�
�ʴ�Ϊ�������¶ȣ����裬���˺����������м���H2SO4 ����ν�ȡ�����ʵ��ӳ���ȡʱ�䣻
��2�������ȡҺ�еĽ���������Ҫ��Cr3+�������Fe3+��Al3+��Ca2+��Mg2+������NaOH��Һʹ��Һ�ʼ��ԣ���ҺPH=4��Fe3+��Al3+ת��Ϊ������ȥ��
�ʴ�Ϊ��Fe3+��
��3�������ӽ�����֬�����������Ǹ����Ӻ�þ���ӡ������ӣ���Ϊ�ڴ�֮ǰ��Fe3+����ȥ��
�ʴ�Ϊ��Ca2+��Al3+��Mg2+��
��4��˫��ˮ��ǿ�����ԣ���������ԭ�Ե����ʣ�Fe2+�л�ԭ�ԣ�Fe2+�ܱ�˫��ˮ����Ϊ�����ӣ���Ӧ�����ӷ���ʽΪ2Fe2++H2O2+2H+=2Fe3++2H2O��
�ʴ�Ϊ��2��1��2��2��2H2O��
��5����������ͼ�е�ת����ϵ�Ͳ����϶�������Ļ�ԭ�Կ�֪�����������ܽ����۸�Ԫ�ػ�ԭ�����۸�Ԫ�أ�����Cr��OH����H2O��5SO4��
�ʴ�Ϊ�������۸�Ԫ�ػ�ԭ�����۸�Ԫ�أ�����Cr��OH����H2O��5SO4��
���� ���⿼�������ӷ���ʽ����ѧ����ʽ����д�����ʵķ����֪ʶ�㣬�ѶȽϴ�ע���������Һ��pHֵ����Һ�е����ӽ��з��룬���ӵ�ԭ���ǣ���ȥ�����Ҳ������µ����ʣ�

| A�� | NaCl��Һ | B�� | BaCl2��Һ | C�� | NaOH��Һ | D�� | ����ʯ��ˮ |
| ѡ�� | �쳣��� | ����ԭ����� |
| A | ��ȡ��Һ�徲�ò��ֲ� | ������ȡ�������϶� |
| B | ��Һ����Һ©���е�Һ�����Ե��� | û�д�Һ©���������������ϰ�����©���ڲ����С��û�ж��� |
| C | �����¶ȼƶ����ﵽ����ֵķе�����ʱ10���ӣ���ƿ��ȴ��Һ�� | �¶ȼ�λ�ô���װ��©����������ˮ������ |
| D | ����Fe2+������KSCN��Һ����Һ�ʺ�ɫ | Fe2+�Ѳ��ֱ�������Fe3+ |
| A�� | A | B�� | B | C�� | C | D�� | D |
 ij��Һ�п��ܺ���H+��Na+��NH4+��Mg2+��Fe3+��Al3+��SO42-�����ӣ��������Һ�м���ijŨ�ȵ�NaOH��Һʱ���������ɳ��������ʵ�����NaOH��Һ������仯��ͼ��ʾ�������йضԸ���Һ���жϲ���ȷ���ǣ�������
ij��Һ�п��ܺ���H+��Na+��NH4+��Mg2+��Fe3+��Al3+��SO42-�����ӣ��������Һ�м���ijŨ�ȵ�NaOH��Һʱ���������ɳ��������ʵ�����NaOH��Һ������仯��ͼ��ʾ�������йضԸ���Һ���жϲ���ȷ���ǣ�������| A�� | ����Һ�п϶����е�������H+��NH4+��Al3+��SO42- | |
| B�� | �϶���������������Mg2+��Fe3+ | |
| C�� | Ҫȷ������Һ�Ƿ���Na+����������ɫ��Ӧʵ�飬����ɫ�Ƿ�Ϊ��ɫ | |
| D�� | ����Һ�п϶����е����ӵ����ʵ���֮��Ϊn��H+����n��NH4+����n��Al3+����n��SO42-��=2��3��1��4 |


 �о�NO2��SO2��CO�ȴ�����Ⱦ����Ĵ���������Ҫ���壮
�о�NO2��SO2��CO�ȴ�����Ⱦ����Ĵ���������Ҫ���壮
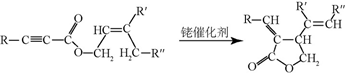


 +CH3CHO$\stackrel{��}{��}$
+CH3CHO$\stackrel{��}{��}$ +H2O��
+H2O��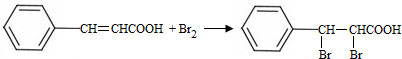 ���Լ�b��NaOH������Һ��
���Լ�b��NaOH������Һ��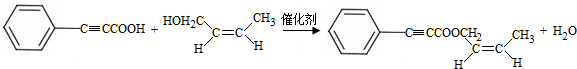 ��
��