��Ŀ����
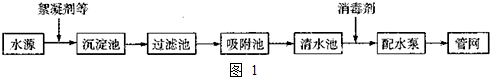
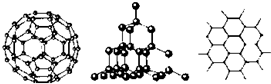
18��C60�����ʯ��ʯī�Ľṹģ����ͼ��ʾ��ʯī����ʾ�����е�һ��ṹ������1��C60�����ʯ��ʯī���ߵĹ�ϵ��ΪB
A��ͬ���칹�� B��ͬ�������� C��ͬϵ�� D��ͬλ��
��2����̬ʱ��C60���ڷ��Ӿ��壨����ӡ�����ԭ�ӡ����ӡ�����C60�����к���˫������Ŀ��30����
��3���辧��Ľṹ�����ʯ���ƣ�1mol�辧���к��й�赥������ĿԼ��2NA������������Ľṹ�൱���ڹ辧��ṹ��ÿ����赥��֮�����1����ԭ�ӣ���������Ŀռ���״�ṹ�У��衢��ԭ���γɵ���С������ԭ����Ŀ��6��
��4��ʯī��״�ṹ��ƽ��ÿ����������ռ�е�̼ԭ������2��
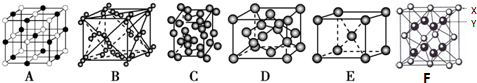
��5�����зֱ��ǿα����Ȼ��ơ��ɱ����⡢���ʯ���ơ�CaF2����ľ���ͼ������ľ���������ͬ����BC��������Ӧ�ı����д����
���� ��1��ͬ��Ԫ�صIJ�ͬ���ʻ���ͬ�������壻
��2�����ݾ��幹�����жϾ������ͣ�
��3�����ݽ��ʯ��С�Ļ�Ϊ��Ԫ���ж϶�������Ŀռ���״�ṹ�У�Si��Oԭ���γɵ���С����Ӧ��6��Siԭ�ӣ�ÿ2��Siԭ��֮����1��Oԭ���ж�Oԭ�ӵ���Ŀ��
��4�����þ�̯�����㣻
��5�������ڷ��Ӿ��壬���þ���ͼ���жϾ���Ĺ��������Դ���������
��� �⣺��1��ͬ��Ԫ�صIJ�ͬ���ʻ���ͬ�������壬C60�����ʯ��ʯī��̼Ԫ�صIJ�ͬ���ʣ�����ͬ�������壻
�ʴ�Ϊ��B��
��2��C60�й������Ƿ��ӣ��������ڷ��Ӿ��壻
��C60�����к���x������κ�y�������Σ�����ŷ���������������ÿ��������������⣬��ÿ�������������������㹲�У�ÿ�����㵥�����������1.5����60�����㹲�������Ϊ60��1.5=90�������ˣ�����ŷ��������д����60+x+y-90=2 �٣�ÿ�������������湲�õģ����ԣ�һ������ε���ռ�е������Ϊ2.5����һ�������ε���ռ�е������Ϊ3������������غ�2.5x+3y=90 �ڣ������٢ڿ��Խ�ã�x=12��y=20��ͨ�����Ϸ���֪����ε�̼�γɹ���˫��������ε�̼�γɹ��۵���������һ����������30��̼̼˫����
�ʴ�Ϊ�����ӣ�30��
��3��C��Si�����ڢ�AԪ�أ����γ�4�����ۼ����ھ������1����ԭ����4����ԭ��ͨ��Si-Siֱ��������ÿ����ԭ�Ӻ���2����������1mol�辧���к��й�赥������ĿԼ��2NA����
���ʯ��С�Ļ�Ϊ��Ԫ������������ṹ�����ʯ�ṹ���ƣ�Si��Oԭ���γɵ���С����Ӧ��6��Siԭ�ӣ��辧��ṹ��ÿ�������Ļ�ѧ��֮�����һ��Oԭ�ӣ���Si��Oԭ���γɵ���С����Oԭ�ӵ���Ŀ��6��
�ʴ�Ϊ��2��6��
��4��ʯī��״�ṹ�У�ÿ��̼ԭ�ӱ������������ι��ã�����ƽ��ÿ����������ռ�е�̼ԭ����=6��$\frac{1}{3}$=2��
�ʴ�Ϊ��2��
��5�������ڷ��Ӿ��壬�ɾ���ͼ��֪��BΪ�ɱ��ľ���ͼ��������Ϊ���ӣ�CΪ��ľ���ͼ��������Ϊ����ӣ�������ľ���������ͬ����BC���ʴ�Ϊ��BC��
���� ���⿼���˾����Ľṹ�������ڿ��龧���ṹ�ķ����ͼ��㣬ע�����þ�̯�����㾧���и���ԭ�Ӹ�������Ŀ�Ѷȴ���ؼ�����ϸ�۲쾧���ṹͼ��

 ��Ӣ���㿨ϵ�д�
��Ӣ���㿨ϵ�д� Ӧ����㲦ϵ�д�
Ӧ����㲦ϵ�д�| A�� | b-a-4 | B�� | b-a-8 | C�� | b+a+8 | D�� | b-a-12 |
| A�� | ����NaCl��aq��$\stackrel{NH_{3}��CO_{2}}{��}$ NaHCO3$\stackrel{��}{��}$Na2CO3 | |
| B�� | Mg3N2$\stackrel{H_{2}O}{��}$Mg��OH��2$\stackrel{��}{��}$MgO | |
| C�� | Mg$��_{����}^{SO_{2}}$MgO | |
| D�� | MgCl2��aq��$\stackrel{����Ũ��}{��}$MgCl2•6H2O$\stackrel{���ڵ��}{��}$Mg |
| A�� | ��ϩ�ͱ�����ʹ��ˮ��ɫ����ɫ��ԭ����ͬ | |
| B�� | 1866�꿭��������˱��ĵ���˫���������������ƽ��ṹ�������˱��IJ������ʣ��������ܽ��͵���ʵ���ڶ��屽ֻ��һ�� | |
| C�� | ���ά��������֬��ABS��֬�����ɸ߷��ӻ�������ɵ����� | |
| D�� | ���������е������������NaOH��Һ��ȥ |
��CH4��g��+2O2��g���TCO2��g��+2H2O��g����H1CH4��g��+2O2��g���TCO2��g��+2H2O��l����H2
��NaOH��aq��+$\frac{1}{2}$H2SO4��Ũ���T$\frac{1}{2}$Na2SO4��aq��+H2O��l����H3
NaOH��aq��+CH3COOH��aq���TCH3COONa��aq��+H2O��l����H4��
| A�� | ��H1����H2����H3����H4 | B�� | ��H1����H2����H3����H4 | C�� | ��H1=��H2����H3����H4 | D�� | ��H1����H2����H3����H4 |
| A�� | Fe2+��Mg2+��Cl-��NO3-�ܴ���������pH=0����Һ�� | |
| B�� | 1 LŨ��Ϊl mol•L-1��NH4Cl��Һ�к���NA��NH4+ | |
| C�� | ��ȥNO�л��е�����NO2���ɽ��������ͨ��ʢ��ˮ��ϴ��ƿ�������ſ������ռ�NO | |
| D�� | ��ӦMnO2+ZnS+2H2SO4�TMnSO4+ZnSO4+S+2H2O�У�ÿ����12.8 g S��ת��0.8mol���� |
 ��
��