��Ŀ����
������A��H��ת����ϵ��ͼ��ʾ��A��һ�־��ô��ᵯ����Ҫ�ɷ֣���֪��

i��A����Է�������Ϊ161��������C��HԪ���⣬��������һ��±��Ԫ�أ�������ֻ����һ������
��������������Cu��OH��2����Һ��1mol C��Ӧ������1mol Cu2O��1molD��
����B1��B2��Ϊͬ���칹�壬B1��Ħ������Ϊ80g/mol��
����G1��G2��Ϊͬ���칹�壬���ߵĺ˴ŴŹ�������ֻ�����������G1����
��֪����
��һ��̼ԭ������������̼̼˫���Ľṹ��-C�TC�TC-�����ȶ���
������������⣺
��1��A�й����ŵ����� ��
��2���١���Ӧ��������ȥ��Ӧ���� ��A�Ľṹ��ʽ ��
��3����Ӧ�ܵĻ�ѧ����ʽ ��
��4��C������Cu��OH��2��Ӧ�ķ���ʽ ��
��5��һ��������H�ܹ����ɸ߷��ӻ�����˷�Ӧ�Ļ�ѧ����ʽ ��
��6����E������ͬ�������Һ�����������ͬ���칹�干�� �֣�д������һ���Ľṹ��ʽΪ ��

i��A����Է�������Ϊ161��������C��HԪ���⣬��������һ��±��Ԫ�أ�������ֻ����һ������
��������������Cu��OH��2����Һ��1mol C��Ӧ������1mol Cu2O��1molD��
����B1��B2��Ϊͬ���칹�壬B1��Ħ������Ϊ80g/mol��
����G1��G2��Ϊͬ���칹�壬���ߵĺ˴ŴŹ�������ֻ�����������G1����
��֪����

��һ��̼ԭ������������̼̼˫���Ľṹ��-C�TC�TC-�����ȶ���
������������⣺
��1��A�й����ŵ�����
��2���١���Ӧ��������ȥ��Ӧ����
��3����Ӧ�ܵĻ�ѧ����ʽ
��4��C������Cu��OH��2��Ӧ�ķ���ʽ
��5��һ��������H�ܹ����ɸ߷��ӻ�����˷�Ӧ�Ļ�ѧ����ʽ
��6����E������ͬ�������Һ�����������ͬ���칹�干��
���㣺�л�����ƶ�
ר�⣺�л���Ļ�ѧ���ʼ��ƶ�
������A��������F����6��Cԭ�ӡ�����1��Brԭ�ӣ���ת����ϵ����֪A�����к���1��Brԭ�ӣ�ȥ��1��Br��ʣ��ʽ��Ϊ161-80=81��̼ԭ�������ĿΪ
=7��9����A�����к���7��̼ԭ�ӡ�9��Hԭ�ӡ�1��Brԭ�ӣ���A�ķ���ʽΪC6H9Br�������������IJ���ֻ��һ�֣���AӦ�ǻ�״����������Ͷ�Ϊ2���ʻ�����1��̼̼˫��������ӽṹ��ֻ��һ��-CH3����AΪ��������Ԫ��״�����A�ij�������IJ���C��Cu��OH��2��Ӧ�ı�����ϵ֪C������ֻ��һ��ȩ������A�м��������е�һ��������̼ԭ���ϣ�A������ȥ��Ӧ���ɵ�B1��B2��Ϊͬ���칹�壬A��Brԭ�Ӳ�����������IJ�����̼ԭ�����ڣ�B2�������������õ�G1��G2��Ϊͬ���칹�壬������3��̼ԭ�ӣ���A��Brԭ����̼̼˫��̼ԭ��֮�����1��̼ԭ�ӣ���A�Ľṹ��ʽΪ 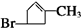 ������������Ϣ��֪��A��������������C����CΪ
������������Ϣ��֪��A��������������C����CΪ ��C����ΪD���������������ӳɷ�Ӧ�����������µõ�E����DΪ
��C����ΪD���������������ӳɷ�Ӧ�����������µõ�E����DΪ ��EΪHOOCCH2CHBrCH2CH��OH��CH3��E����������Ӧ����F����FΪ
��EΪHOOCCH2CHBrCH2CH��OH��CH3��E����������Ӧ����F����FΪ ��B1��B2��A����ȥ��������ʽΪC6H8��B2��������õ�G1��G2��Ϊͬ���칹�壬���ߵĺ˴Ź�������ֻ�����������G1������B2Ϊ
��B1��B2��A����ȥ��������ʽΪC6H8��B2��������õ�G1��G2��Ϊͬ���칹�壬���ߵĺ˴Ź�������ֻ�����������G1������B2Ϊ ��G1ΪOHC-CH2-CHO��G2ΪOHCCOCH3��HΪHOOCCH��OH��CH3���ݴ˽��
��G1ΪOHC-CH2-CHO��G2ΪOHCCOCH3��HΪHOOCCH��OH��CH3���ݴ˽��
| 81 |
| 12 |
 ������������Ϣ��֪��A��������������C����CΪ
������������Ϣ��֪��A��������������C����CΪ ��C����ΪD���������������ӳɷ�Ӧ�����������µõ�E����DΪ
��C����ΪD���������������ӳɷ�Ӧ�����������µõ�E����DΪ ��EΪHOOCCH2CHBrCH2CH��OH��CH3��E����������Ӧ����F����FΪ
��EΪHOOCCH2CHBrCH2CH��OH��CH3��E����������Ӧ����F����FΪ ��B1��B2��A����ȥ��������ʽΪC6H8��B2��������õ�G1��G2��Ϊͬ���칹�壬���ߵĺ˴Ź�������ֻ�����������G1������B2Ϊ
��B1��B2��A����ȥ��������ʽΪC6H8��B2��������õ�G1��G2��Ϊͬ���칹�壬���ߵĺ˴Ź�������ֻ�����������G1������B2Ϊ ��G1ΪOHC-CH2-CHO��G2ΪOHCCOCH3��HΪHOOCCH��OH��CH3���ݴ˽��
��G1ΪOHC-CH2-CHO��G2ΪOHCCOCH3��HΪHOOCCH��OH��CH3���ݴ˽�����
�⣺A��������F����6��Cԭ�ӡ�����1��Brԭ�ӣ���ת����ϵ����֪A�����к���1��Brԭ�ӣ�ȥ��1��Br��ʣ��ʽ��Ϊ161-80=81��̼ԭ�������ĿΪ
=7��9����A�����к���7��̼ԭ�ӡ�9��Hԭ�ӡ�1��Brԭ�ӣ���A�ķ���ʽΪC6H9Br�������������IJ���ֻ��һ�֣���AӦ�ǻ�״����������Ͷ�Ϊ2���ʻ�����1��̼̼˫��������ӽṹ��ֻ��һ��-CH3����AΪ��������Ԫ��״�����A�ij�������IJ���C��Cu��OH��2��Ӧ�ı�����ϵ֪C������ֻ��һ��ȩ������A�м��������е�һ��������̼ԭ���ϣ�A������ȥ��Ӧ���ɵ�B1��B2��Ϊͬ���칹�壬A��Brԭ�Ӳ�����������IJ�����̼ԭ�����ڣ�B2�������������õ�G1��G2��Ϊͬ���칹�壬������3��̼ԭ�ӣ���A��Brԭ����̼̼˫��̼ԭ��֮�����1��̼ԭ�ӣ���A�Ľṹ��ʽΪ  ������������Ϣ��֪��A��������������C����CΪ
������������Ϣ��֪��A��������������C����CΪ ��C����ΪD���������������ӳɷ�Ӧ�����������µõ�E����DΪ
��C����ΪD���������������ӳɷ�Ӧ�����������µõ�E����DΪ ��EΪHOOCCH2CHBrCH2CH��OH��CH3��E����������Ӧ����F����FΪ
��EΪHOOCCH2CHBrCH2CH��OH��CH3��E����������Ӧ����F����FΪ ��B1��B2��A����ȥ��������ʽΪC6H8��B2��������õ�G1��G2��Ϊͬ���칹�壬���ߵĺ˴Ź�������ֻ�����������G1������B2Ϊ
��B1��B2��A����ȥ��������ʽΪC6H8��B2��������õ�G1��G2��Ϊͬ���칹�壬���ߵĺ˴Ź�������ֻ�����������G1������B2Ϊ ��G1ΪOHC-CH2-CHO��G2ΪOHCCOCH3��HΪHOOCCH��OH��CH3��
��G1ΪOHC-CH2-CHO��G2ΪOHCCOCH3��HΪHOOCCH��OH��CH3��
��1��������������֪��AΪ �����еĹ����ŵ�����Ϊ��̼̼˫������ԭ�ӣ��ʴ�Ϊ��̼̼˫������ԭ�ӣ�
�����еĹ����ŵ�����Ϊ��̼̼˫������ԭ�ӣ��ʴ�Ϊ��̼̼˫������ԭ�ӣ�
��2���١���Ӧ��������ȥ��Ӧ���Ǣݣ�A�Ľṹ��ʽΪ�� ���ʴ�Ϊ���ݣ�
���ʴ�Ϊ���ݣ� ��
��
��3����Ӧ�ܵĻ�ѧ����ʽ��HOOCCH2CHBrCH2CH��OH��CH3
 +H2O��
+H2O��
�ʴ�Ϊ��HOOCCH2CHBrCH2CH��OH��CH3
 +H2O��
+H2O��
��4�� ������Cu��OH��2����Һ ��Ӧ�ķ���ʽΪ��
������Cu��OH��2����Һ ��Ӧ�ķ���ʽΪ�� +2Cu��OH��2+NaOH
+2Cu��OH��2+NaOH
 +Cu2O��+3H2O��
+Cu2O��+3H2O��
�ʴ�Ϊ�� +2Cu��OH��2+NaOH
+2Cu��OH��2+NaOH
 +Cu2O��+3H2O��
+Cu2O��+3H2O��
��5��H��һ�����������ɸ߾���ķ�Ӧ����ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��6��E[HOOCCH2CHBrCH2CH��OH��CH3]��ͬ���칹�������������E������ͬ�������Һ����������������������E��ͬ���칹��Ϊ�� ��
�� ��
��
�ʴ�Ϊ��4�� ��
�� ����һ�֣�
����һ�֣�
| 81 |
| 12 |
 ������������Ϣ��֪��A��������������C����CΪ
������������Ϣ��֪��A��������������C����CΪ ��C����ΪD���������������ӳɷ�Ӧ�����������µõ�E����DΪ
��C����ΪD���������������ӳɷ�Ӧ�����������µõ�E����DΪ ��EΪHOOCCH2CHBrCH2CH��OH��CH3��E����������Ӧ����F����FΪ
��EΪHOOCCH2CHBrCH2CH��OH��CH3��E����������Ӧ����F����FΪ ��B1��B2��A����ȥ��������ʽΪC6H8��B2��������õ�G1��G2��Ϊͬ���칹�壬���ߵĺ˴Ź�������ֻ�����������G1������B2Ϊ
��B1��B2��A����ȥ��������ʽΪC6H8��B2��������õ�G1��G2��Ϊͬ���칹�壬���ߵĺ˴Ź�������ֻ�����������G1������B2Ϊ ��G1ΪOHC-CH2-CHO��G2ΪOHCCOCH3��HΪHOOCCH��OH��CH3��
��G1ΪOHC-CH2-CHO��G2ΪOHCCOCH3��HΪHOOCCH��OH��CH3����1��������������֪��AΪ
 �����еĹ����ŵ�����Ϊ��̼̼˫������ԭ�ӣ��ʴ�Ϊ��̼̼˫������ԭ�ӣ�
�����еĹ����ŵ�����Ϊ��̼̼˫������ԭ�ӣ��ʴ�Ϊ��̼̼˫������ԭ�ӣ���2���١���Ӧ��������ȥ��Ӧ���Ǣݣ�A�Ľṹ��ʽΪ��
 ���ʴ�Ϊ���ݣ�
���ʴ�Ϊ���ݣ� ��
����3����Ӧ�ܵĻ�ѧ����ʽ��HOOCCH2CHBrCH2CH��OH��CH3

 +H2O��
+H2O���ʴ�Ϊ��HOOCCH2CHBrCH2CH��OH��CH3

 +H2O��
+H2O����4��
 ������Cu��OH��2����Һ ��Ӧ�ķ���ʽΪ��
������Cu��OH��2����Һ ��Ӧ�ķ���ʽΪ�� +2Cu��OH��2+NaOH
+2Cu��OH��2+NaOH| �� |
 +Cu2O��+3H2O��
+Cu2O��+3H2O���ʴ�Ϊ��
 +2Cu��OH��2+NaOH
+2Cu��OH��2+NaOH| �� |
 +Cu2O��+3H2O��
+Cu2O��+3H2O����5��H��һ�����������ɸ߾���ķ�Ӧ����ʽΪ��
 ��
���ʴ�Ϊ��
 ��
����6��E[HOOCCH2CHBrCH2CH��OH��CH3]��ͬ���칹�������������E������ͬ�������Һ����������������������E��ͬ���칹��Ϊ��
 ��
�� ��
���ʴ�Ϊ��4��
 ��
�� ����һ�֣�
����һ�֣�
���������⿼���л�����ƶϺͺϳɣ�����ʱע������ú������Ϣ���������������ϵķ����ƶϣ���Ҫѧ���������չ����ŵ�������ת������ѧ�����������нϸߵ�Ҫ��ȷ��A�Ľṹ�ǹؼ�����Ŀ��Ϊ�ۺϣ��ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ
����������ȫ�ۻ�������������������Һ�У����ᷢ����Ӧ���ǣ�������
| A��ϡ���� | B��ϡ���� |
| C������ͭ | D���������� |
�����й��������ʵ�˵��������ǣ�������
| A�����ȶ��ԣ�HCl��HBr��HI |
| B��ԭ�Ӱ뾶��Na��Mg��Al |
| C����ԭ�ԣ�PH3��H2S��HCl |
| D���������������ClO-��HCO3-��SiO32- |
���л�ѧ����ʽ�����ӷ���ʽ�У���ȷ���ǣ�������
A����Ũ������4-��-1-�������ȷ�����ȥ��Ӧ��BrCH2CH2CH2OH
| |||
B��ˮ���ᣨ ���м���NaHCO3��Һ�� ���м���NaHCO3��Һ�� +2HCO3�� +2HCO3�� +2CO2��+2H2O +2CO2��+2H2O | |||
| C��������������Һ�еμ����������Һ��pH=7��Ba2++OH-+H++SO42-=BaSO4��+H2O | |||
| D���ú����Ƽ�Ƶ�NaHCO3��Na++NH3+CO2+H2O=NaHCO3��+NH4+ |
�������������еı仯���漰������ԭ��Ӧ���ǣ�������
| A�����ʳ��������͵����ϳɰ� |
| B������ú����Ƽ�ƴ��� |
| C�����᳧�ýӴ����������� |
| D���ȼ�õ�ⱥ��ʳ��ˮ���ռ� |


 ������Դ�뻷������Խ��Խ�����ǹ�ע��̼һ��ѧ��C1��ѧ����Ϊ�о����ȵ㣮��̼һ��ѧ�����Ե���̼��CO��CO2��CH4��CH3OH�Ⱥ�һ��̼ԭ�ӵ�����Ϊԭ�Ϻϳɹ�ҵ��Ʒ�Ļ�ѧ�빤�գ�
������Դ�뻷������Խ��Խ�����ǹ�ע��̼һ��ѧ��C1��ѧ����Ϊ�о����ȵ㣮��̼һ��ѧ�����Ե���̼��CO��CO2��CH4��CH3OH�Ⱥ�һ��̼ԭ�ӵ�����Ϊԭ�Ϻϳɹ�ҵ��Ʒ�Ļ�ѧ�빤�գ�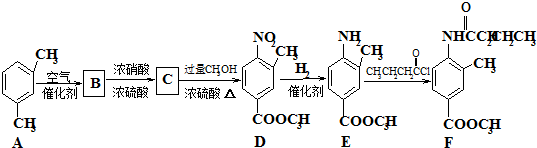
 ��һ�������¿�ˮ��Ϊ
��һ�������¿�ˮ��Ϊ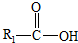 ��R2-NH2����F��ǿ��ͳ�ʱ����������·���ˮ�ⷴӦ�Ļ�ѧ����ʽ��
��R2-NH2����F��ǿ��ͳ�ʱ����������·���ˮ�ⷴӦ�Ļ�ѧ����ʽ��