��Ŀ����
�㾫�� ����ʳƷ��ҩƷ����ױƷ�ȷ���Ӧ�ù㷺��������A�ϳ��㾫�����������л����·�����£�
����ʳƷ��ҩƷ����ױƷ�ȷ���Ӧ�ù㷺��������A�ϳ��㾫�����������л����·�����£�

��֪����Aֻ������һ�ȴ���B��C
��
�ش��������⣺
��1���� ��Ϊͬ���칹����ǣ� ������ţ�
��Ϊͬ���칹����ǣ� ������ţ�

��2��D���ʵ�����Ϊ��
��3����E�ϳ�F�Ļ�ѧ����ʽΪ�� ����Ӧ����Ϊ
��4����ҵ��CH3CH2COONa�� ֱ�Ӻϳɸ��㾫����ѧ����ʽΪ ��
ֱ�Ӻϳɸ��㾫����ѧ����ʽΪ ��
��5����G��ij��һԪȩ�ͱ�Ҫ�����Լ�Ϊԭ�Ϻϳ� ��д���ϳ�·�ߣ�ע����������Ҫ��д��ѧ����ʽ�� ��
��д���ϳ�·�ߣ�ע����������Ҫ��д��ѧ����ʽ�� ��
 ����ʳƷ��ҩƷ����ױƷ�ȷ���Ӧ�ù㷺��������A�ϳ��㾫�����������л����·�����£�
����ʳƷ��ҩƷ����ױƷ�ȷ���Ӧ�ù㷺��������A�ϳ��㾫�����������л����·�����£�
��֪����Aֻ������һ�ȴ���B��C
��

�ش��������⣺
��1����
 ��Ϊͬ���칹����ǣ�
��Ϊͬ���칹����ǣ�
��2��D���ʵ�����Ϊ��
��3����E�ϳ�F�Ļ�ѧ����ʽΪ��
��4����ҵ��CH3CH2COONa��
 ֱ�Ӻϳɸ��㾫����ѧ����ʽΪ
ֱ�Ӻϳɸ��㾫����ѧ����ʽΪ��5����G��ij��һԪȩ�ͱ�Ҫ�����Լ�Ϊԭ�Ϻϳ�
 ��д���ϳ�·�ߣ�ע����������Ҫ��д��ѧ����ʽ��
��д���ϳ�·�ߣ�ע����������Ҫ��д��ѧ����ʽ�����㣺�л���ĺϳ�
ר�⣺�л���Ļ�ѧ���ʼ��ƶ�
����������H�ͱ��״�����������Ӧ������Ľṹ��ʽ֪��H��CH3CH2COOH��G����������Ӧ����CH3CH2COOH����G�Ľṹ��ʽΪCH3CH2CHO��C���������Ƶ�ˮ��Һ����ȡ����Ӧ���ɴ���Ȼ����������CH3CH2CHO��A����ȡ����Ӧ����C����C�Ľṹ��ʽΪCH3CH2CH2Cl��A����������A�Ľṹ��ʽΪCH3CH2CH3��A�ڹ��������·���ȡ����Ӧ����B����B�Ľṹ��ʽΪCH3CHClCH3��B������ȥ��ӦȻ�����ӳɷ�Ӧ����D��D�Ľṹ��ʽΪCH2BrCHBrCH3��D���������Ƶ�ˮ��Һ����ȡ����Ӧ����E��E�Ľṹ��ʽΪCH2OHCH��OH��CH3��E�ͶԱ������ᷢ��������Ӧ����F��F�Ľṹ��ʽΪ ���ݴ˷������
���ݴ˷������
 ���ݴ˷������
���ݴ˷���������
�⣺����H�ͱ��״�����������Ӧ������Ľṹ��ʽ֪��H��CH3CH2COOH��G����������Ӧ����CH3CH2COOH����G�Ľṹ��ʽΪCH3CH2CHO��C���������Ƶ�ˮ��Һ����ȡ����Ӧ���ɴ���Ȼ����������CH3CH2CHO��A����ȡ����Ӧ����C����C�Ľṹ��ʽΪCH3CH2CH2Cl��A����������A�Ľṹ��ʽΪCH3CH2CH3��A�ڹ��������·���ȡ����Ӧ����B����B�Ľṹ��ʽΪCH3CHClCH3��B������ȥ��ӦȻ�����ӳɷ�Ӧ����D��D�Ľṹ��ʽΪCH2BrCHBrCH3��D���������Ƶ�ˮ��Һ����ȡ����Ӧ����E��E�Ľṹ��ʽΪCH2OHCH��OH��CH3��E�ͶԱ������ᷢ��������Ӧ����F��F�Ľṹ��ʽΪ ��
��
��1����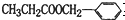 ��Ϊͬ���칹����Ǣ٢ڢܣ��ʴ�Ϊ���٢ڢܣ�
��Ϊͬ���칹����Ǣ٢ڢܣ��ʴ�Ϊ���٢ڢܣ�
��2��D�Ľṹ��ʽΪCH2BrCHBrCH3����������1��2-������飬�ʴ�Ϊ��1��2-������飻
��3��ͨ�����Ϸ���֪��E�ϳ�F�Ļ�ѧ����ʽΪnCH3CH��OH��CH2��OH��+n
 +��2n-1��H2O����Ӧ����Ϊ���۷�Ӧ��
+��2n-1��H2O����Ӧ����Ϊ���۷�Ӧ��
�ʴ�Ϊ��nCH3CH��OH��CH2��OH��+n
 +��2n-1��H2O�����۷�Ӧ��
+��2n-1��H2O�����۷�Ӧ��
��4����ҵ��CH3CH2COONa�� ����ȡ����Ӧ�ϳ��㾫����ѧ����ʽΪCH3CH2COONa+
����ȡ����Ӧ�ϳ��㾫����ѧ����ʽΪCH3CH2COONa+ ��
�� +NaCl��
+NaCl��
�ʴ�Ϊ��CH3CH2COONa+ ��
�� +NaCl��
+NaCl��
��5��GΪCH3CH2CHO��������Ϣ��Ӧ����G��2���ӵ�HCHO������Ӧ����CH3C��CH2OH��2CHO�����������ӳɵõ� ������Ϊ��
��������
CH3CH2CHO
CH3CH��CH2OH��CHO
CH3C��CH2OH��2CHO
 ��
��
�ʴ�Ϊ��CH3CH2CHO
CH3CH��CH2OH��CHO
CH3C��CH2OH��2CHO
 ��
��
 ��
����1����
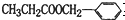 ��Ϊͬ���칹����Ǣ٢ڢܣ��ʴ�Ϊ���٢ڢܣ�
��Ϊͬ���칹����Ǣ٢ڢܣ��ʴ�Ϊ���٢ڢܣ���2��D�Ľṹ��ʽΪCH2BrCHBrCH3����������1��2-������飬�ʴ�Ϊ��1��2-������飻
��3��ͨ�����Ϸ���֪��E�ϳ�F�Ļ�ѧ����ʽΪnCH3CH��OH��CH2��OH��+n

| Ũ���� |
| �� |
 +��2n-1��H2O����Ӧ����Ϊ���۷�Ӧ��
+��2n-1��H2O����Ӧ����Ϊ���۷�Ӧ���ʴ�Ϊ��nCH3CH��OH��CH2��OH��+n

| Ũ���� |
| �� |
 +��2n-1��H2O�����۷�Ӧ��
+��2n-1��H2O�����۷�Ӧ����4����ҵ��CH3CH2COONa��
 ����ȡ����Ӧ�ϳ��㾫����ѧ����ʽΪCH3CH2COONa+
����ȡ����Ӧ�ϳ��㾫����ѧ����ʽΪCH3CH2COONa+ ��
�� +NaCl��
+NaCl���ʴ�Ϊ��CH3CH2COONa+
 ��
�� +NaCl��
+NaCl����5��GΪCH3CH2CHO��������Ϣ��Ӧ����G��2���ӵ�HCHO������Ӧ����CH3C��CH2OH��2CHO�����������ӳɵõ�
 ��������
��������CH3CH2CHO
| ϡNaOH |
| HCHO |
| ϡNaOH |
| HCHO |
| H2 |
| ���� |
 ��
���ʴ�Ϊ��CH3CH2CHO
| ϡNaOH |
| HCHO |
| ϡNaOH |
| HCHO |
| H2 |
| ���� |
 ��
��
���������⿼�����л�����ƶϣ��ɱ��ᱽ�����Ľṹ��ʽ�������Ƶķ������з��������ȷ�л���Ĺ����ż��������ǽⱾ��ؼ����Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
����������Ԫ��A��B��C��D��ԭ�Ӱ뾶���μ�С������A�ĵ�����һ����Ҫ�İ뵼����ϣ�B�ĵ��ʳ���������ˮ������C�ǽ��������ܶ������н�������С��D��һ����̬�⻯����ʹʪ��ĺ�ɫʯ����ֽ������������˵��������ǣ�������
| A��D����̬�⻯���A����̬�⻯���ȶ� |
| B��A�ij�����������һ���������������ˮ��Ӧ����һ������ |
| C��B������������Ӧ��ˮ������������ǿ�ĺ����� |
| D������C����������ˮ��Ӧ����һ������ |
�����������벻�����ֻ�ѧ���ʣ�����˵������ȷ���ǣ�������
| A������Ҫͨ����ѧ��Ӧ���ܴӺ�ˮ�л��ʳ�κ͵�ˮ |
| B��DZˮͧ�ڽ�������¿��ù������ƹ��� |
| C���������ռ���Һ��ʯ���鷴Ӧ���ܵõ����������� |
| D�����ӵ��Ρ������߸�ţ�̡���������Ӫ��Ʒ����ʳƷ�еĵ⡢�ơ�����ָ���� |
 600��ʱ����һ�̶��ݻ����ܱ������У������������������Ϸ�����Ӧ��2SO2��g��+O2��g��?2SO3��g����H��0����Ӧ������SO2��O2��SO3���ʵ����ı仯��ͼ������˵��������ǣ�������
600��ʱ����һ�̶��ݻ����ܱ������У������������������Ϸ�����Ӧ��2SO2��g��+O2��g��?2SO3��g����H��0����Ӧ������SO2��O2��SO3���ʵ����ı仯��ͼ������˵��������ǣ�������| A����Ӧ�ӿ�ʼ����һ��ƽ��ʱ�����������ת����Ϊ20% |
| B��10��15 min��20��25min��ȣ�ǰ�ߵĻ�ѧ��Ӧ���ʴ� |
| C����Ӧ������20minʱ�����߷����仯����Ϊͨ�������� |
| D����Ӧ���е�10min��15 min�����߱仯ԭ��һ����������ѹǿ |
��ѧ�г�������ͼ����һ�����ֶ���������ͻ��ʵ��װ�õ�Ҫ�㣬����ز�����ѧ���̵�ԭ���������йػ�ѧͼ����ֵ�������ȷ���ǣ�������
A�� �ⶨһ��ʱ��������H2�ķ�Ӧ���� |
B�� ��ȡ�����еĵ� |
C�� ������ȼ�ղ�����SO2 |
D�� ֤���ǽ����ԣ�Cl��C��Si |

 ���ⶨijNaOH��Һ�����ʵ���Ũ�ȣ��������ʵ���Ũ��Ϊ0.1000mol?L-1 HCl����Һ�����к͵ζ����÷�̪��ָʾ������
���ⶨijNaOH��Һ�����ʵ���Ũ�ȣ��������ʵ���Ũ��Ϊ0.1000mol?L-1 HCl����Һ�����к͵ζ����÷�̪��ָʾ������