��Ŀ����
��1��4�������ȫȼ��ʱ�ų�37ǧ���������÷�Ӧ���Ȼ�ѧ����ʽ�� ��
��2�����ܱ������м���һ������N2O4����һ���¶���N2O4��g��?2NO2��g������ʱ���������ܶ�Ϊ��ͬ������H2�ܶȵ�36.8����N2O4�ķֽ����� ��
��3����1L�ܱ������У�����amolN2��bmolH2����һ���¶���N2+3H2?2NH3���ﵽƽ�⣬�����л�ʣ��c molN2����ƽ��ʱN2��ת������ ��������H2��ƽ��Ũ���� ��
��2�����ܱ������м���һ������N2O4����һ���¶���N2O4��g��?2NO2��g������ʱ���������ܶ�Ϊ��ͬ������H2�ܶȵ�36.8����N2O4�ķֽ�����
��3����1L�ܱ������У�����amolN2��bmolH2����һ���¶���N2+3H2?2NH3���ﵽƽ�⣬�����л�ʣ��c molN2����ƽ��ʱN2��ת������
���㣺�Ȼ�ѧ����ʽ,��ѧƽ��ļ���
ר�⣺�����������������
��������1�������Ȼ�ѧ����ʽ����д������
��2��ƽ����������ܶ�����ͬ������H2�ܶȵ�36.8��������ƽ���������ƽ����Է�������Ϊ36.8��2=73.6��������ʼʱΪ1molN2O4����ƽ��ʱ�������Ϊ
=1.25mol����Ϸ�Ӧ�ķ���ʽ���ò��������㣻
��3�����������л�ʣ��c molN2��������ĵ�N2�����ʵ������ټ����N2��ת���ʣ���������ʽ������ƽ��ʱH2��ƽ��Ũ�ȣ�
��2��ƽ����������ܶ�����ͬ������H2�ܶȵ�36.8��������ƽ���������ƽ����Է�������Ϊ36.8��2=73.6��������ʼʱΪ1molN2O4����ƽ��ʱ�������Ϊ
| 92g/mol |
| 73.6g/mol |
��3�����������л�ʣ��c molN2��������ĵ�N2�����ʵ������ټ����N2��ת���ʣ���������ʽ������ƽ��ʱH2��ƽ��Ũ�ȣ�
���
�⣺��1��4g�����ȫȼ�����ɶ����������壬�ų�37kJ������������32g�����ȫȼ�����ɶ����������壬�ų�296kJ�����������Ȼ�ѧ����ʽΪ��S��s��+O2��g���TSO2��g����H=-296KJ/mol��
�ʴ�Ϊ��S��s��+O2��g���TSO2��g����H=-296KJ/mol��
��2��ƽ����������ܶ�����ͬ������H2�ܶȵ�36.8��������ƽ���������ƽ����Է�������Ϊ36.8��2=73.6��������ʼʱΪ1molN2O4����ƽ��ʱ�������Ϊ
=1.25mol����ֽ��N2O4�����ʵ���Ϊx��
N2O4��g��?2NO2��g�����������ʵ�������ֵ
1 2 1
x 1.25mol-1mol=0.25mol
x=0.25mol��
N2O4�ķֽ���Ϊ
=25%��
�ʴ�Ϊ��25%��
��3�����ݷ�Ӧ����ʽN2+3H2 ?2NH3
��ʼ a b 0
ת�� a-c 3��a-c�� 2��a-c��
ƽ�� c b-3��a-c�� 2��a-c��
��ƽ��ʱN2��ת����=
��100%��
������H2��ƽ��Ũ��=
=��b-3a+3c��mol/L
�ʴ�Ϊ��
��100%����b-3a+3c��mol/L��
�ʴ�Ϊ��S��s��+O2��g���TSO2��g����H=-296KJ/mol��
��2��ƽ����������ܶ�����ͬ������H2�ܶȵ�36.8��������ƽ���������ƽ����Է�������Ϊ36.8��2=73.6��������ʼʱΪ1molN2O4����ƽ��ʱ�������Ϊ
| 92g/mol |
| 73.6g/mol |
N2O4��g��?2NO2��g�����������ʵ�������ֵ
1 2 1
x 1.25mol-1mol=0.25mol
x=0.25mol��
N2O4�ķֽ���Ϊ
| 0.25 |
| 1 |
�ʴ�Ϊ��25%��
��3�����ݷ�Ӧ����ʽN2+3H2 ?2NH3
��ʼ a b 0
ת�� a-c 3��a-c�� 2��a-c��
ƽ�� c b-3��a-c�� 2��a-c��
��ƽ��ʱN2��ת����=
| a-c |
| a |
������H2��ƽ��Ũ��=
| b-3(a-c) |
| 1 |
�ʴ�Ϊ��
| a-c |
| a |
���������⿼���Ȼ�ѧ����ʽ����д����ѧƽ��ļ��㣬������ѧ�����������ͼ��������Ŀ��飬ע���������ܶȼ����������ƽ����Է�����������������ʽ���Խ����⣬��Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
�ϳɰ���ҵ�Թ��ú���ᷢչ������Ҫ�����壮�������ϳɰ���Ҫ��20��50MPa�ĸ�ѹ��500�����ҵĸ����£���������ý��Ϊ����������ƽ�������еĺ����Խ��٣����Ӣ�����ոԴ�ѧ�Ļ�ѧ��ʹ����һ����Ϊ transһFe��DMeOPrPE��2���´������ڳ����ºϳɰ�����Ӧ����ʽ�ɱ�ʾΪN2+3H2?2NH3���й�˵����ȷ���ǣ�������
| A���������ϳɰ������ȷ�Ӧ���·��ϳɰ��Ƿ��ȷ�Ӧ |
| B���·��ϳɰ�����Ҫ�ڸ��������£��ɽ�Լ������Դ�����з�չǰ�� |
| C���·��ϳɰ����ڳ����½�������Ϊ����Ҫ���ѻ�ѧ�� |
| D���´��������˷�Ӧ����Ҫ��������ʹƽ�������ƶ� |
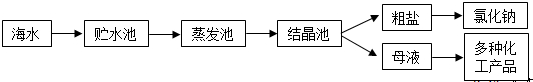
�����������Ʒֱ�Ͷ���������ʵ�ˮ��Һ�У�������ų�������Һ����������ǣ�������
| A��HCl |
| B��K2SO4 |
| C��FeCl3 |
| D��NaCl |
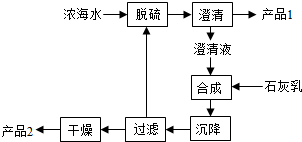
 ��ҵ���Ի�����Ϊԭ�ϣ����ýӴ����������ᣮ��ش��������⣺
��ҵ���Ի�����Ϊԭ�ϣ����ýӴ����������ᣮ��ش��������⣺
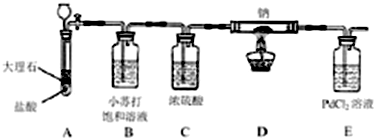
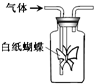 ��ͼ����ƿ������ֽ�۳ɵ�ֽ������������һ����Һ��ͨ��ij�������ʵ������Ԥ���ֽ������ɫ�仯��һ�µ��ǣ�������
��ͼ����ƿ������ֽ�۳ɵ�ֽ������������һ����Һ��ͨ��ij�������ʵ������Ԥ���ֽ������ɫ�仯��һ�µ��ǣ�������