��Ŀ����
��1���������л�ѧ����ʽ�ش����⣺
SiO2+2C+2Cl2
SiCl4+2CO
����˫���ű�ʾ����Ӧ�е���ת�Ƶķ������Ŀ
���ڸ÷�Ӧ�У���ԭ���� ��
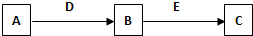
��2��A��B��C��D��E�dz�����������ʣ�������ת����ϵ����ȥ��������Ʒ����
����AΪNa��BΪNaOH��CΪNa2CO3����д��Aת��ΪB�Ļ�ѧ����ʽ ��
����AΪ��̬���ʣ�BΪƯ�۵���Ҫ�ɷ�֮һ��C����Ư���ԣ���д��Aת��ΪB�Ļ�ѧ����ʽ ��д����ѧ����ʽ֤��C�������� ��������ɵ���A��Ԫ��ԭ�ӽṹʾ��ͼ ��
SiO2+2C+2Cl2
| ||
����˫���ű�ʾ����Ӧ�е���ת�Ƶķ������Ŀ
���ڸ÷�Ӧ�У���ԭ����
��2��A��B��C��D��E�dz�����������ʣ�������ת����ϵ����ȥ��������Ʒ����

����AΪNa��BΪNaOH��CΪNa2CO3����д��Aת��ΪB�Ļ�ѧ����ʽ
����AΪ��̬���ʣ�BΪƯ�۵���Ҫ�ɷ�֮һ��C����Ư���ԣ���д��Aת��ΪB�Ļ�ѧ����ʽ
���㣺������ƶ�,������ԭ��Ӧ
ר�⣺�ƶ���,������ԭ��Ӧר��
��������1����ӦSiO2+2C+2Cl2
SiCl4+2CO�У�CԪ�ػ��ϼ���0�����ߵ�+2�ۣ���������CΪ��ԭ����ClԪ�ػ��ϼ���0�۽��͵�-1�ۣ�����ԭ��Cl2Ϊ��������
��2������AΪNa��BΪNaOH��CΪNa2CO3����ת����ϵ��֪DΪH2O��EΪCO2��
��AΪ��̬���ʣ�BΪƯ�۵���Ч�ɷݣ�BΪCa��ClO��2����A
B��֪��AΪCl2��DΪCa��OH��2��C����Ư���ԣ���ת����ϵ��֪��CΪHClO��
| ||
��2������AΪNa��BΪNaOH��CΪNa2CO3����ת����ϵ��֪DΪH2O��EΪCO2��
��AΪ��̬���ʣ�BΪƯ�۵���Ч�ɷݣ�BΪCa��ClO��2����A
| D |
���
�⣺��1���ٷ�ӦSiO2+2C+2Cl2
SiCl4+2CO�У�CԪ�ػ��ϼ���0�����ߵ�+2�ۣ���������CΪ��ԭ����ClԪ�ػ��ϼ���0�۽��͵�-1�ۣ�����ԭ��Cl2Ϊ������������ת�Ʒ������Ŀ�ɱ�ʾΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
�ڷ�Ӧ��CԪ�ػ��ϼ����ߣ���������Ϊ��ԭ�����ʴ�Ϊ��C��
��2������AΪNa��BΪNaOH��CΪNa2CO3����ת����ϵ��֪DΪH2O��EΪCO2��Aת��ΪB�Ļ�ѧ����ʽΪ2Na+2H2O=2NaOH+H2����
�ʴ�Ϊ��2Na+2H2O=2NaOH+H2����
��AΪ��̬���ʣ�BΪƯ�۵���Ч�ɷݣ�BΪCa��ClO��2����A
B��֪��AΪCl2��DΪCa��OH��2��C����Ư���ԣ���ת����ϵ��֪��CΪHClO���������������Ʒ�Ӧ�����Ȼ��ơ����������ˮ����Ӧ����ʽΪ��2Cl2+2Ca��OH��2=CaCl2+Ca��ClO��2+2H2O��HClOΪ���ᣬ���Ա�̼�������ɷ���Ca��ClO��2+CO2+2H2O=CaCO3+2HClO��A�����ԭ��ΪCl��ԭ�Ӻ�����3�����Ӳ㣬����������Ϊ7��ԭ�ӽṹʾ��ͼΪ ��
��
�ʴ�Ϊ��2Cl2+2Ca��OH��2=CaCl2+Ca��ClO��2+2H2O��Ca��ClO��2+CO2+2H2O=CaCO3+2HClO�� ��
��
| ||
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
���ڷ�Ӧ��CԪ�ػ��ϼ����ߣ���������Ϊ��ԭ�����ʴ�Ϊ��C��
��2������AΪNa��BΪNaOH��CΪNa2CO3����ת����ϵ��֪DΪH2O��EΪCO2��Aת��ΪB�Ļ�ѧ����ʽΪ2Na+2H2O=2NaOH+H2����
�ʴ�Ϊ��2Na+2H2O=2NaOH+H2����
��AΪ��̬���ʣ�BΪƯ�۵���Ч�ɷݣ�BΪCa��ClO��2����A
| D |
 ��
���ʴ�Ϊ��2Cl2+2Ca��OH��2=CaCl2+Ca��ClO��2+2H2O��Ca��ClO��2+CO2+2H2O=CaCO3+2HClO��
 ��
��
����������������ת������ʽ����Na��Cl��Ԫ�ص��ʼ��仯����֮����ת����ϵ����ѧ�������д��ּ�ڿ���ѧ����Ԫ�ػ�����֪ʶ�������ճ̶ȣ�ע�⣨2���з�����������ϲ�ֹһ�֣�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
��״���½�VLHCl�ܽ���1Lˮ�У�ˮ���ܶȽ���Ϊ1
����������Һ���ܶ�Ϊ
����������Ϊw������Ũ��Ϊc
�������й�ϵ�в���ȷ���ǣ�������
| g |
| mL |
| ��g |
| mL |
| mol |
| L |
A����=
| ||
B��c=
| ||
C��V=
| ||
D����=
|
 ��ʢ��ϡ������ձ��з����õ������ӵ���Ƭ��ͭƬ������������ȷ���ǣ�������
��ʢ��ϡ������ձ��з����õ������ӵ���Ƭ��ͭƬ������������ȷ���ǣ�������| A�������ķ�ӦʽΪ��Cu2++2e-=Cu |
| B����Ƭ���ܽ⣬��ӦʽΪ��Fe-3e-=Fe3+ |
| C�������ǵ�������ĵ缫 |
| D��ͭƬΪ�����������˻�ԭ��Ӧ���ɹ۲쵽���ݲ��� |
����H++OH-=H2O����ʾ�Ļ�ѧ��Ӧ�ǣ�������
| A��NaOH��Һ����ᷴӦ |
| B��KOH��Һ��ϡ���ᷴӦ |
| C��Ba��OH��2��Һ��ϡ���ᷴӦ |
| D��ʯ��ˮ��ϡ���ᷴӦ |
���и���ֵ�ȷ���ǣ�������
| A��S��T��P�£�1mol�κ���������Ϊ22.4L |
| B������ӵ³���Ϊ6.02��1023/mol |
| C����NA��������ӵ���������1mol H2������ΪNA�� |
| D��S��T��P�£�0.5mol CO2�����Ϊ11.2L |
 ��1��ԭ��ط�Ӧͨ���Ƿ��ȷ�Ӧ���������Ͽ���Ƴ�ԭ��صĻ�ѧ��Ӧ��
��1��ԭ��ط�Ӧͨ���Ƿ��ȷ�Ӧ���������Ͽ���Ƴ�ԭ��صĻ�ѧ��Ӧ�� ��1��һ���¶��£���1L�ܱ������м���1mol HI��g��������2HI��g��?H2��g��+I2��g����Ӧ��H2���ʵ�����ʱ��ı仯��ͼ��ʾ��0��2min�ڵ�ƽ����Ӧ����v��HI��=
��1��һ���¶��£���1L�ܱ������м���1mol HI��g��������2HI��g��?H2��g��+I2��g����Ӧ��H2���ʵ�����ʱ��ı仯��ͼ��ʾ��0��2min�ڵ�ƽ����Ӧ����v��HI��= ����������ԭ��Ӧ��2Fe3++Cu=Cu2++2Fe2+��Ƶ�ԭ�����ͼ��ʾ����ش��������⣺
����������ԭ��Ӧ��2Fe3++Cu=Cu2++2Fe2+��Ƶ�ԭ�����ͼ��ʾ����ش��������⣺