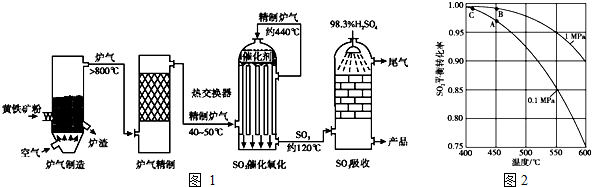
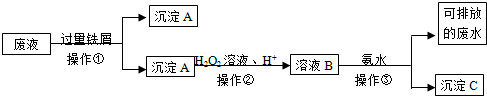
��Ŀ����
A��B��C�Ǻ˵����������������ֶ���������Ԫ�أ�AԪ�ص�ԭ�Ӻ���ֻ��1�����ӣ�BԪ�ص�ԭ�Ӱ뾶����������������С�ģ�B������������Ӧˮ����Ļ�ѧʽΪHBO3��CԪ��ԭ�ӵ������������ȴ�����4����
��1��B�����ڱ��е�λ��
��2����ҵ�ϳ�BA3�Ļ�ѧ����ʽΪ
��3��BA3������ͬ�������ĸ��������� �� ��
��4����֪BA3��A2C�Ļ�����Լ��ԣ��������ӷ���ʽ��ʾԭ�� ��
��1��B�����ڱ��е�λ��
��2����ҵ�ϳ�BA3�Ļ�ѧ����ʽΪ
��3��BA3������ͬ�������ĸ���������
��4����֪BA3��A2C�Ļ�����Լ��ԣ��������ӷ���ʽ��ʾԭ��
���㣺λ�ýṹ���ʵ����ϵӦ��
ר�⣺Ԫ����������Ԫ�����ڱ�ר��
������AԪ�ص�ԭ�Ӻ���ֻ��1�����ӣ���AΪHԪ�أ�BԪ�ص�ԭ�Ӱ뾶����������������С�ģ�B������������Ӧˮ����Ļ�ѧʽΪHBO3����B������ϼ�Ϊ+5�ۣ�λ�����ڱ��ڢ�A�壬ӦΪNԪ�أ�CԪ��ԭ�ӵ������������ȴ�����4������ԭ�Ӻ�������Ų�Ϊ2��6��ӦΪOԪ�أ�����ԭ�ӵ���ɡ����ʽ�������ɵĵݱ���ɽ����⣮
���
�⣺���ݷ�����֪��AΪ�⣬BΪ����CΪ����
��1��BΪ����N�����ڱ��ڶ����ڵ�VA�壬�ʴ�Ϊ���ڶ����ڵ�VA�壻
��2����ҵ�����õ����������ڸ��¸�ѹ�����������·�Ӧ���ʷ�Ӧ�Ļ�ѧ��Ӧ����ʽΪ��N2+3H2
2NH3���ʴ�Ϊ��N2+3H2
2NH3��
��3��BA3ΪNH3��NH3��10�����ӣ�����10�����ӵĸ���������OH-��H3O+��NH4+��NH2-�ȣ��ʴ�Ϊ��OH-��H3O+��NH4+��
��4��NH3��H2O��Ӧ����һˮ�ϰ���һˮ�ϰ���������������������Һ��ʾ���ԣ����ӷ�Ӧ����ʽΪ��NH3+H2O?NH4++OH-���ʴ�Ϊ��NH3+H2O?NH4++OH-��
��1��BΪ����N�����ڱ��ڶ����ڵ�VA�壬�ʴ�Ϊ���ڶ����ڵ�VA�壻
��2����ҵ�����õ����������ڸ��¸�ѹ�����������·�Ӧ���ʷ�Ӧ�Ļ�ѧ��Ӧ����ʽΪ��N2+3H2
| ���¸�ѹ |
| ���� |
| ���¸�ѹ |
| ���� |
��3��BA3ΪNH3��NH3��10�����ӣ�����10�����ӵĸ���������OH-��H3O+��NH4+��NH2-�ȣ��ʴ�Ϊ��OH-��H3O+��NH4+��
��4��NH3��H2O��Ӧ����һˮ�ϰ���һˮ�ϰ���������������������Һ��ʾ���ԣ����ӷ�Ӧ����ʽΪ��NH3+H2O?NH4++OH-���ʴ�Ϊ��NH3+H2O?NH4++OH-��
���������⿼��Ԫ��λ�ýṹ���ʵ����ϵӦ�ã���Ŀ�Ѷ��еȣ�ע����ȷ�ƶ�Ԫ�ص�����Ϊ������Ĺؼ���

��ϰ��ϵ�д�
 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�
�����Ŀ
����ʵ��װ��ͼ��ȷ���ܴﵽʵ��Ŀ���ǣ�������
A�� ʵ�����Ʊ���������ϩ |
B�� ʯ�ͷ��� |
C�� ��֤���ᡢ̼�ᡢ��������ǿ�� |
D�� ʵ�����Ʊ���Ȳ������������ |
 ��һ�������£����ڷ�ӦmA��g��+nB��g��?cC��g��+dD��g����C���ʵ�Ũ�ȣ�c�����¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ�������ж���ȷ���ǣ�������
��һ�������£����ڷ�ӦmA��g��+nB��g��?cC��g��+dD��g����C���ʵ�Ũ�ȣ�c�����¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ�������ж���ȷ���ǣ�������| A����H��0��S��0 |
| B����H��0��S��0 |
| C����S��0��H��0 |
| D����H��0��S��0 |
1Lij��Һ�к��е�����������ö��Ե缫������Һ������·����3mol e-ͨ��ʱ�����Ե��ʱ��Һ����ı仯���缫������ܴ��ڵ��ܽ���������˵����ȷ���ǣ�������
| ���� | Cu2+ | Al3+ | NO | Cl- |
| ���ʵ���Ũ�ȣ�mol/L�� | 1 | 1 | a | 1 |
| A��������Һ��pH=0 |
| B��a=3 |
| C����������1.5 mol Cl2 |
| D�����������Ľ�����ͭ���� |