��Ŀ����
7��ʵ����������ͼʵ��װ�ý����йػ�ѧʵ�飬�ش��������⣺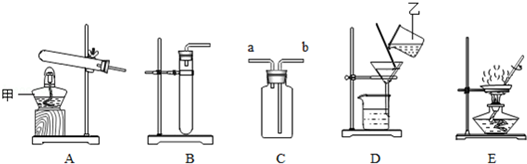
��1��д��ͼ�������ס��ҵ����ƣ��ƾ��ƣ����ձ���
��2���ó���ˮ��װ��C�ռ����������������a���a����b������ͨ�룬ʵ�����ø��������ȡ����������װ�ÿ�ѡ��ͼ�е�A�����ţ���д���÷�Ӧ�Ļ�ѧ����ʽ2KMnO4$\frac{\underline{\;��\;}}{\;}$K2MnO4+MnO2+O2����
��3��ʵ������ȡ������̼�Ļ�ѧ����ʽ��CaCO3+2HCl=CaCl2+CO2��+H2O����װ��C�ռ�������̼������ʱ��ȼ��ľ��Ӧ����a���a����b�����ˣ����������̼���Լ��dz���ʯ��ˮ��
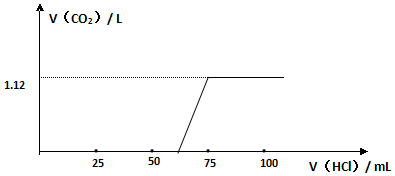
���� ��1������������������������
��2��������ˮ�����ռ�����ʱ������Ҫ�Ӷ̹ܽ���ˮ�ӳ��ܳ���ʵ�����ø��������ȡ�����Ļ�ѧ����ʽΪ2KMnO4$\frac{\underline{\;��\;}}{\;}$K2MnO4+MnO2+O2�����ݴ˿�֪�Ǽ��ȹ�������ȡ���壬�ݴ˷�������װ�õ�ѡ��
��3��ʵ�����������ʯ��ʯ��Ӧ����ȡ������̼�����ڶ�����̼������ˮ���ܶȱȿ������������ſ��������ռ�������̼���ó���ʯ��ˮ�����������̼���壮
��� �⣺��1���������������ο�֪����Ϊ�ƾ��ƣ���Ϊ�ձ����ʴ�Ϊ���ƾ��ƣ��ձ���
��2��������ˮ�����ռ�����ʱ������Ҫ�Ӷ̹ܽ���ˮ�ӳ��ܳ�����������a����ʵ�����ø��������ȡ�����Ļ�ѧ����ʽΪ2KMnO4$\frac{\underline{\;��\;}}{\;}$K2MnO4+MnO2+O2�����ݴ˿�֪�Ǽ��ȹ�������ȡ���壬���Թܿ�Ҫ������б����Ӧѡ��װ��A���ʴ�Ϊ��a��A�� 2KMnO4$\frac{\underline{\;��\;}}{\;}$K2MnO4+MnO2+O2����
��3��ʵ�����������ʯ��ʯ��Ӧ����ȡ������̼����ѧ����ʽΪ��CaCO3+2HCl=CaCl2+CO2��+H2O�����ڶ�����̼������ˮ���ܶȱȿ������������ſ��������ռ�������̼����������̼��b�ܽ���������a�ܳ���������ʱ��ȼ��ľ��Ӧ����a�ˣ�ʵ����ͨ���ó���ʯ��ˮ�����������̼���壮
�ʴ�Ϊ��CaCO3+2HCl=CaCl2+CO2��+H2O�� a�� ����ʯ��ˮ��
���� ���⿼���������ʵ�����Ʒ���ʵ��װ�õ�ѡ���ѶȲ���ע��Ӧ���ݷ�Ӧ������������Ʊ�װ�ã�����������������������ռ�������

 ��Ӣ���㿨ϵ�д�
��Ӣ���㿨ϵ�д� ijѧ������ֽ�۳�һֻֽ��������ֽ����������ij���Լ�����������̨�ϣ���ȡһֻʢ��ij����Һ���ձ�������ֽ�������·�����ͼ������һ��������ְ�ɫֽ�����ϵ�����Һת��Ϊ��ɫ��������ֽ�����ϵ��Լ���С�ձ��е���Һ�ǣ�������
ijѧ������ֽ�۳�һֻֽ��������ֽ����������ij���Լ�����������̨�ϣ���ȡһֻʢ��ij����Һ���ձ�������ֽ�������·�����ͼ������һ��������ְ�ɫֽ�����ϵ�����Һת��Ϊ��ɫ��������ֽ�����ϵ��Լ���С�ձ��е���Һ�ǣ�������| A | B | C | D | |
| ֽ�����ϵ�����Һ | ʯ�� | ��̪ | ��̪ | ʯ�� |
| С�ջ��е���Һ | Ũ��ˮ | Ũ��ˮ | ����������Һ | Ũ���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ���£�0.1mol•L-1��CH3COOH��Һ�У�c��CH3COOH����c��CH3COO-�� | |
| B�� | 1L 0.1mol•L-1�ģ�NH4��2Fe��SO4��2��Һ�У�c��SO${\;}_{4}^{2-}$����c��NH${\;}_{4}^{+}$����c��Fe2+����c��H+����c��OH-�� | |
| C�� | ���£�0.1mol•L-1��CH3COONa��NaOH��Na2CO3������Һ��pH��С��˳��Ϊ��NaOH��CH3COONa��Na2CO3 | |
| D�� | ��0.01mol•L-1��NaHSO4��Һ�еμ�NaOH��Һ������ʱ��c��SO${\;}_{4}^{2-}$����c��Na+����c��OH-��=c��H+�� |
| �������� | ʵ���������� | ��Ӧ���ӷ���ʽ��ѧ����ʽ |
| FeCl2��ͨ��Cl2 | ��Һ��� | 2Fe2++Cl2=2Fe3+ |
| Fe��OH��2���ÿ��� | ��ɫ����Ѹ�ٱ�Ϊ����ɫ�����ձ�Ϊ���ɫ | 4Fe��OH��2+O2+4H2O=4Fe��OH��3 |
| KI��Һ��ͨ������������CCl4���� | ��Һ��Ϊ��ɫ���������Ȼ�̼��ֲ㣬�����Ȼ�̼������ɫ | Cl2+2I-=2Cl-+I2 |
| ������Һ�л����μ�Na[Al��OH��4]��Һ������ | �������������ɰ�ɫ���� | [Al��OH��4]-+4H+=Al3++4H2O��3[Al��OH��4]-+Al3+=4Al��OH��3�� |
| ���鷽�� | ������ | ��ɫ�� | ���巨 |
| ���� | ��Ӧ���г����������ܽ� | ��Ӧ������ɫ�仯 | ��Ӧ����������� |
| A�� | NH4+-���巨 | B�� | I--������ | ||
| C�� | Na+-��ɫ�� | D�� | CO32--���巨�ͳ����� |
 Ϊά������ѪҺ�е�Ѫ�Ǻ������ڸ�������Һʱ��ͨ����������ע��Һ����ͼΪ������Һ�õ������ǣ���ѧʽΪC6H12O6��ע��Һ��ǩ�ϵIJ��փ��ݣ���ע��Һ���������ǵ�ˮ��Һ�����Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
Ϊά������ѪҺ�е�Ѫ�Ǻ������ڸ�������Һʱ��ͨ����������ע��Һ����ͼΪ������Һ�õ������ǣ���ѧʽΪC6H12O6��ע��Һ��ǩ�ϵIJ��փ��ݣ���ע��Һ���������ǵ�ˮ��Һ�����Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺