��Ŀ����
20������ʵ���������ȷ���ǣ�����������ʣ���ƻ��Ӧ��ʱ�Ż�ԭ�Լ�ƿ
�������������ҺʱҪ�ò��������Ͻ���
�۳�ȡ�׳����ҩƷ������ڲ��������г���
������ֽ������������ʱ����������ֽ��ˮ��ϴ�����壬�۲���ֽ��ɫ�仯
�ݽ���������������Һ�ij���ͷ�ι���������������Һ���ټ�����Һ���Ƶ�����������
��ˮ����Ӧ��ʢ��ĥ�ڲ��������Լ�ƿ�У�
| A�� | �٢ۢ� | B�� | �ڢۢ� | C�� | �٢ڢۢ� | D�� | �٢ڢۢ� |
���� �ٽ����ƻ����������е�ˮ��������Ӧ��
�ڲ������������ʹ��Һ���Ⱦ��ȣ�
���׳����ҩƷӦ�÷���С�ձ��Ȳ��������г�����
������ֽ������������ʱ����������������ֽ��ˮ��ϴ������۲���ֽ��ɫ�ı仯��
�������������ױ������е�����������
��ˮ�����ܹ�������ƿ�ں�ƿ�����һ��
��� �⣺�ٽ����ƻ����������е�ˮ��������Ӧ��ʣ���ҩƷҪ�Ż�ԭƿ����ô�����ڰ�ȫ�������ʢ���ȷ��
�������������Һʱ��Ϊ��ʹ��Һ���Ⱦ��ȣ���Ҫ�ò��������Ͻ��裬�ʢ���ȷ��
�۳�ȡ�׳����ҩƷ��Ϊ�˱���ҩƷ�����⣬����ʱ������ڲ��������г������ʢ���ȷ��
�ܲⶨ��Һ��pHʱ����պ�д�����Һ�IJ������������ֽ���в�������ֽ��ɫ���������ɫ���Ƚ���ȷ����Һ��pH�����߽���ֽ��ʪ��ճ���������ϣ������������壬����ֽ��ɫ���������ɫ���Ƚ���ȷ����Һ��pH���ʢܴ���
�������������ױ������е�����������Ӧ����Һ���£��ʢ���ȷ��
��ˮ��������ճ�ԣ��ܽ�����ƿ�ں�ƿ�����һ�𣬲����ò��������ʢ���
��ѡC��
���� ���⿼�黯ѧʵ�鷽�������ۣ��漰������ѧ�Լ��ı��棬��Ŀ�ѶȲ������ǻ���������Ŀ��飬Ҳ�Ǹ߿��еij������㣮����Ĺؼ�����ȷ������ѧ�Լ������ʣ������������ʺͻ�ѧ���ʣ�Ȼ��������Ե�ѡ�ɣ�

| A�� |  ������������10��̼ԭ�Ӵ���ͬһƽ�� ������������10��̼ԭ�Ӵ���ͬһƽ�� | |
| B�� | �����ʺ���֬�����ڸ߷��ӻ����һ����������ˮ�� | |
| C�� | �����ʵ����ı��ͱ�������ȫȼ�����ĵ�������������� | |
| D�� | ���ⶨ�Ҷ����ͱ���ɵĻ������������������Ϊ8%����˻������̼������������84% |
| A�� | KClO3 | B�� | CH3Cl | C�� | CCl4 | D�� | ��ˮ |
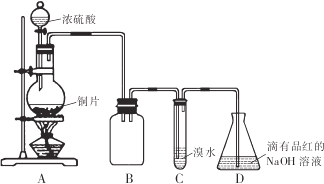
��ش��������⣺
��1��װ��B�������Ƿ���������ȫƿ��
��2�����װ��C��Ŀ������֤SO2�Ļ�ԭ�ԣ�װ��C�з�����Ӧ�����ӷ���ʽ��SO2+Br2+2H2O=4H++SO42-+2Br-��װ��D��NaOHȫ��ת��ΪNaHSO3�ı�־����Һ�ɺ�ɫ��Ϊ��ɫ��
��3����NaHSO3��Һ�м���NaCl0��Һʱ����Ӧ�����ֿ��ܵ������
I��HSO3-��ClO-ǡ�÷�Ӧ
��NaClO����
��NaClO����
��ͬѧ�ֱ�ȡ���������Һ���Թ��У�ͨ������ʵ��ȷ���÷�Ӧ������һ�������������±�������֪���ԣ�H2SO3��H2CO3��HCl0��
| ��� | ʵ ����� | �� �� | �� �� |
| �� | �Ӽ�С��CaCO3���� | �����ݲ��� | I��� |
| �� | �μ���������KI��Һ���� | �� | |
| �� | �μ�������ˮ���� | �� | |
| �� | �μ���������KMn04��Һ���� | ��ҺΪ��ɫ |
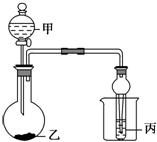 ����ͼ��ʾװ�ý�������ʵ�飬�ܹ۲쵽��Ӧ���ó���Ӧʵ����۵��ǣ�������
����ͼ��ʾװ�ý�������ʵ�飬�ܹ۲쵽��Ӧ���ó���Ӧʵ����۵��ǣ�������| ѡ�� | �� | �� | �� | ʵ������ | ʵ����� |
| A | Ũ���� | ʯ��ʯ | NaAlO2��Һ | ��������ɫ���ݣ������ȳ��ֳ�������ʧ | ������������̼�� |
| B | Ũ���� | ���� | ��ˮ | �������DZ��ɫ��������ˮ��ɫ | Ũ���������ˮ�ԡ������� |
| C | ϡ���� | Na2SO3 | Ba��NO3��2��Һ | ��������ɫ���ݣ����г��ְ�ɫ���� | SO2������Ա��ξ������ɰ�ɫ���� |
| D | Ũ���� | Na2CO3 | Na2SiO3��Һ | ��������ɫ���ݣ����г��ְ�ɫ���� | ���ԣ����̼����� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | 6.75 g | B�� | 4.05g | C�� | 2.70 g | D�� | 1.80 g |
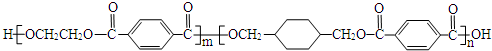
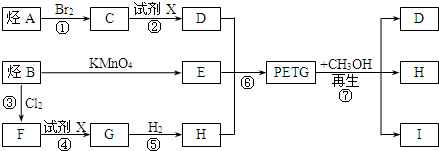
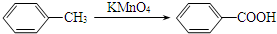
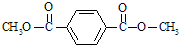 ��
�� ��
��