��Ŀ����
7����A��B��C��D��E 5�ֻ��������A��B��C��D����Ԫ�صĻ����F��һ�����壬�ڱ�״���¶Կ���������ܶ���1.103���������з�Ӧ����A+NaOH��D+H2O
��B��A+H2O
��C+NaOH ����������B+NaCl
��E+H2O��NaOH+F
��C+D+H2O��B
��1��A��B��C��D��E��ѧʽ�ֱ��ǣ�AAl2O3��BAl��OH��3��CAlCl3��DNaAlO2��ENa2O2
��2��д���ܵ����ӷ���ʽ��2Na2O2+2H2O�T4Na++4OH-+O2����
���� F��һ�����壬��״��������ڿ������ܶ�Ϊ1.103������Է�������Ϊ1.103��29=32��FӦΪO2����EΪNa2O2��A��B��C��D�Ǻ���Ԫ�صĻ�����ɷ�Ӧ�ڿ�֪AΪAl2O3��BΪAl��OH��3����CΪAlCl3��DΪNaAlO2����϶�Ӧ���ʵ����ʽ����⣮
��� �⣺��1��F��һ�����壬��״��������ڿ������ܶ�Ϊ1.103������Է�������Ϊ1.103��29=32��FӦΪO2����EΪNa2O2��A��B��C��D�Ǻ���Ԫ�صĻ�����ɷ�Ӧ�ڿ�֪AΪAl2O3��BΪAl��OH��3����CΪAlCl3��DΪNaAlO2��
�ʴ�Ϊ��Al2O3��Al��OH��3��AlCl3��NaAlO2��Na2O2��
��2����Ӧ�ܵ����ӷ���ʽΪ��2Na2O2+2H2O�T4Na++4OH-+O2����
�ʴ�Ϊ��2Na2O2+2H2O�T4Na++4OH-+O2����
���� ���⿼��������ƶϣ��漰Na��AlԪ�ػ�����������ת����ע���������ܶ�ȷ��F���ٽ�����ʵ�ת����ϵ�ƶϣ��������ճ���Ԫ�ػ���������ʣ���Ŀ�ѶȲ���

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
15�������й��Ȼ�ѧ����ʽ��������ȷ���ǣ�������
| A�� | ��֪�����ȼ����Ϊ890.3kJ/mol�������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪCH4��g��+2O2��g���T2CO2��g��+2H2O��g����H=-890.3 kJ/mol | |
| B�� | ��֪C��ʯī��s���TC�����ʯ��s����H��0������ʯ��ʯī�ȶ� | |
| C�� | ��֪�к���Ϊ��H=-57.3 kJ/mol����1molϡ���������ϡNaOH��Һ��Ӧ�ķ�Ӧ�Ⱦ����к��� | |
| D�� | ��֪S��g��+O2��g���TSO2��g����H1��S��s��+O2��g���TSO2��g����H2�����H1����H2 |
19��������ʵ�У�������ɳ����ԭ�������͵��ǣ�������
| A�� | ��H 2��g����I 2��g����HI��g����ɵĻ������ƽ����ϵ��ѹ����ɫ���� | |
| B�� | ���õ���ˮ�����ϡ���� | |
| C�� | ��FeCl 3��Һ�м������۷�ֹ�������� | |
| D�� | �������������SO 2��O 2��Ӧ��SO 3 |
16��ijͬѧ�����в�������100mL0.200mol•L-1Na2CO3��Һ����ش��й����⣮
���۰������������Ƶ�Na2CO3��Һ��Ũ�Ȳ��ǣ�ѡ��ǡ����ǡ���0.200mol•L-1����������Ϊ��ͬѧû��ϴ���ձ��Ͳ�������
| ʵ�鲽�� | �й����� |
| ��1����������Na2CO3������ | ��ҪNa2CO3������Ϊ2.12g�� |
| ��2������Na2CO3���� | ����������Ӧ�õ�����Ҫ�����Ƿ�����ƽ�������ƽ�� |
| ��3����Na2CO3����100mL�ձ��У�������������ˮ | Ϊ�˼ӿ��ܽ����ʣ�����ȡ�Ĵ�ʩ���ò��������裮 |
| ��4�����ձ��е���Һת��������A���Ѽ�鲻©ˮ���� | ����ת��Na2CO3��ҺǰӦ����Һ��ȴ�����£� ������A��100mL����ƿ�� ��Ϊ��ֹ��Һ������Ӧ��ȡ�Ĵ�ʩ���ò����������� |
| ��5��������A�м�����ˮ���̶��� | �ڽ��д˲���ʱӦע��������Ǽ�����ˮ������ƿ�е�Һ��ӽ��̶���1-2cm�������ý�ͷ�ιܵμ�����Һ�İ�Һ��������̶������У� |
| ��6��ҡ�ȡ�װƿ������B�������ࡢ���� | ����B������ǩ�� |

 +NaOH��
+NaOH�� +CH3OH��
+CH3OH�� ���������ͨ����ȥ��Ӧ���ɻ�����I�Ļ�ѧ����ʽΪ
���������ͨ����ȥ��Ӧ���ɻ�����I�Ļ�ѧ����ʽΪ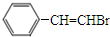 +NaOH$��_{��}^{��}$
+NaOH$��_{��}^{��}$ +NaBr+H2O��ע����Ӧ��������
+NaBr+H2O��ע����Ӧ��������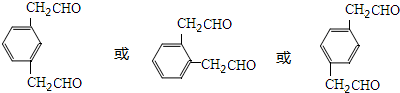
 ����Ľṹ��ʽΪ
����Ľṹ��ʽΪ
