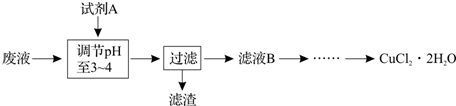
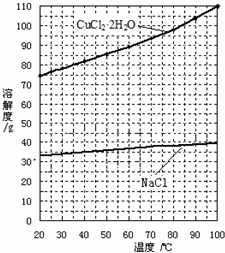
��Ŀ����
 ����ͼʾװ����ȡ���������������ƾ��Ƶ���ͼ�о�����ȥ������գ�
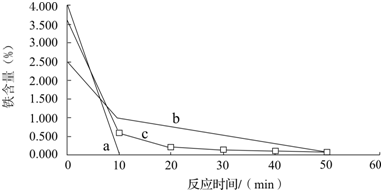
����ͼʾװ����ȡ���������������ƾ��Ƶ���ͼ�о�����ȥ������գ���1���Թ�a����Ҫ����Ũ���ᡢ��������Ҵ���2mL����ȷ����˳�������
��2��д���Թ�a�и÷�Ӧ��ѧ����ʽ
��3��ʵ���м����Թܣ�
�ٿ�ʼʱҪС����ȵ�Ŀ���ǣ�
�ڼ���һ��ʱ����Ϊ�����������ڵ�Ŀ���ǣ�
��4���Թ�b�м��б���Na2CO3��Һ���������ǣ�
��5���Թ�b�е����ܲ�����Һ���µ�Ŀ���ǣ�
���㣺������������ȡ
ר�⣺�л���Ļ�ѧ���ʼ��ƶ�
��������1��Ũ�����ܶȴ�Ӧ��Ũ������뵽�Ҵ��У��Է���Һ�ɽ��������ӷ�����ȴ���ټ������
Һ�����Ҫ�����Ƭ����ֹ���У�
��2��������Ӧ�ı���Ϊ�����ǻ��������⣬�÷�Ӧ��������������ˮ����Ϊ���淴Ӧ��
��3���ټ����Ҵ�������Ļӷ����ڼ�ʱ��������������������������ƽ�����������������ķ����ƶ����ۼ��ٸ���Ӧ������
��4������̼������Һ�����ᷴӦ��ȥ���ᡢͬʱ���������������ܽ�ȣ����ڷֲ㣻
��5�����ռ�������ˮ������ʱ��������������ˮ�У�������������ˮ������װ����ѹǿ���罵�ͣ�������ѹѹ��Һ����룬������������
Һ�����Ҫ�����Ƭ����ֹ���У�
��2��������Ӧ�ı���Ϊ�����ǻ��������⣬�÷�Ӧ��������������ˮ����Ϊ���淴Ӧ��
��3���ټ����Ҵ�������Ļӷ����ڼ�ʱ��������������������������ƽ�����������������ķ����ƶ����ۼ��ٸ���Ӧ������
��4������̼������Һ�����ᷴӦ��ȥ���ᡢͬʱ���������������ܽ�ȣ����ڷֲ㣻
��5�����ռ�������ˮ������ʱ��������������ˮ�У�������������ˮ������װ����ѹǿ���罵�ͣ�������ѹѹ��Һ����룬������������
���
�⣺��1��Ϊ��ֹ��Һ�ɽ���Ӧ���ܶȴ��Һ����뵽�ܶ�С��Һ���У������ӷ�����ȴ���ټ������ᣬҺ�������Ҵ��е�ͣ�����Ҫ�����Ƭ����ֹ���У�
�ʴ�Ϊ���ȼ����Ҵ���Ȼ���ҡ���Թܱ���������Ũ�����ȴ���ټӱ�������Թ�a�м��뼸����ʯ�������Ƭ����
��2��������Ӧ�ı���Ϊ�����ǻ��������⣬�������Ҵ���Ũ���������¼��ȷ���������Ӧ��������������ˮ���÷�ӦΪ���淴ӦΪ��CH3COOH+C2H5OH
CH3COOC2H5+H2O��
�ʴ�Ϊ��CH3COOH+C2H5OH
CH3COOC2H5+H2O��
��3�����Ҵ��������ӷ����ʿ�ʼʱС����ȣ��ʴ�Ϊ�������Ҵ�������Ļӷ���
�ڼ���һ��ʱ����Ϊ�����������ڵ�Ŀ���Ǽ�ʱ��������������������������ƽ�����������������ķ����ƶ����ʴ�Ϊ����ʱ��������������������
�۸÷�ӦΪ���ȷ�Ӧ�������¶�������ƽ�����������������ķ����ƶ����ٸ���Ӧ������
�ʴ�Ϊ������ƽ�����������������ķ����ƶ������ٸ���Ӧ������
��4���Ʊ���������ʱ���ñ���̼������Һ��Ŀ�����кͻӷ����������ᣬʹ֮ת��Ϊ����������ˮ�У��ܽ�ӷ��������Ҵ�����������������ˮ�е��ܽ�ȣ����ڷֲ�õ�����
�ʴ�Ϊ���������������������������������ʺ��Ҵ�����������������ˮ�е��ܽ�ȣ�
��5���������뱥��̼������Һ�У�����������Ҵ����ڱ���̼������Һ������װ����ѹǿ���罵�ͣ�������ѹѹ��Һ����룬������������
�ʴ�Ϊ����ֹ������
�ʴ�Ϊ���ȼ����Ҵ���Ȼ���ҡ���Թܱ���������Ũ�����ȴ���ټӱ�������Թ�a�м��뼸����ʯ�������Ƭ����
��2��������Ӧ�ı���Ϊ�����ǻ��������⣬�������Ҵ���Ũ���������¼��ȷ���������Ӧ��������������ˮ���÷�ӦΪ���淴ӦΪ��CH3COOH+C2H5OH
| Ũ���� |
| �� |
�ʴ�Ϊ��CH3COOH+C2H5OH
| Ũ���� |
| �� |
��3�����Ҵ��������ӷ����ʿ�ʼʱС����ȣ��ʴ�Ϊ�������Ҵ�������Ļӷ���
�ڼ���һ��ʱ����Ϊ�����������ڵ�Ŀ���Ǽ�ʱ��������������������������ƽ�����������������ķ����ƶ����ʴ�Ϊ����ʱ��������������������
�۸÷�ӦΪ���ȷ�Ӧ�������¶�������ƽ�����������������ķ����ƶ����ٸ���Ӧ������
�ʴ�Ϊ������ƽ�����������������ķ����ƶ������ٸ���Ӧ������
��4���Ʊ���������ʱ���ñ���̼������Һ��Ŀ�����кͻӷ����������ᣬʹ֮ת��Ϊ����������ˮ�У��ܽ�ӷ��������Ҵ�����������������ˮ�е��ܽ�ȣ����ڷֲ�õ�����
�ʴ�Ϊ���������������������������������ʺ��Ҵ�����������������ˮ�е��ܽ�ȣ�
��5���������뱥��̼������Һ�У�����������Ҵ����ڱ���̼������Һ������װ����ѹǿ���罵�ͣ�������ѹѹ��Һ����룬������������
�ʴ�Ϊ����ֹ������
���������⿼���������������Ʊ������ʱ��ע��������Ӧ��ԭ���ͱ���̼������Һ�����ã���Ŀ�ѶȲ���

��ϰ��ϵ�д�
 ����С״Ԫ��������������ϵ�д�
����С״Ԫ��������������ϵ�д�
�����Ŀ
�����������ӻ������⣬����˵������ȷ���ǣ�������
| A�����೬��̼�ŷż���������Ͷ���������ŷ����γ��������Ҫԭ�� |
| B��װ��װ�����еļ�ȩ�������������������ʶ������������Ⱦ |
| C��úȼ��ʱ����������ʯ��ʯ���Լ��ٷ����еĶ��������ŷ� |
| D���ҹ���ʵʩ�����������������ɫ��Ⱦ����һ���̶��ϵõ���Ч���� |
�������ӷ���ʽ��ѧ��Ӧ����ʽ��������ʵ�������ȷ���ǣ�������
| A����2molSO3����ͨ��һ�ܱ������У���ƽ�������QkJ��������÷�Ӧ���Ȼ�ѧ����ʽΪ��2SO3��g��?2SO2��g��+O2��g����H=+QkJ/mol |
| B��������SO2����ͨ��NaClO��Һ�У�SO2+H2O+2ClO-=SO32-+2HClO |
| C��NH4Al��SO4��2��Һ�м���Ba��OH��2��ҺʹSO42-��ȫ������Al3++2SO42-+2Ba2++4OH-=AlO2-+2BaSO4��+2H2O |
| D������0.4molFeBr2����Һ��ͨ��0.3molCl2��ַ�Ӧ��4Fe2++2Br-+3Cl2=4Fe3++6Cl-+Br2 |
���з��������е�ԭ�Ӷ����������Ϊ8���ӽṹ���ǣ�������
| A��BCl3 |
| B��COCl2 |
| C��SF6 |
| D��SiH4 |
һ���۵��ӹ���Ϊ2s22p5��Ԫ�أ������й�������������ȷ���ǣ�������
| A��ԭ������Ϊ9 |
| B���縺��������Ԫ�������� |
| C��ԭ�Ӱ뾶��ͬ����Ԫ������С�� |
| D����һ������������Ԫ�������� |
 ijѧ�������һ����֤���ʻ�ѧ���ʵ�ʵ��װ�ã���ͼ������۲��װ��ͼ������������⣺
ijѧ�������һ����֤���ʻ�ѧ���ʵ�ʵ��װ�ã���ͼ������۲��װ��ͼ������������⣺