��Ŀ����
������Ԫ��W��X��Y��Z��ԭ�������������ӡ�m��n��p������ЩԪ����ɵĶ�Ԫ�����W2��X2��Z2�ֱ���Ԫ��W��X��Z�ĵ��ʡ���֪��
I��һ��������ij�ܱ������пɷ�����Ӧ��aX2+bW2 cm����Ӧ���������ʵ�Ũ�ȱ仯���£�
cm����Ӧ���������ʵ�Ũ�ȱ仯���£�
| X2 | W2 | m |
��ʼŨ��/mol��L��1 | 0.4 | 0.4 | 0 |
ƽ��Ũ��/mol��L��1 | 0.3 | 0.1 | 0.2 |
II�����ǿɷ������·�Ӧ��2m(g)+3Z(g)=6n(g)+X2(g)��4n(g)+Y2(g) 2p(l)+2Z2(g)��
2p(l)+2Z2(g)��
����˵����ȷ����
A��ԭ�Ӱ뾶��W��X��Y B��a��b��c=3:1:2
C��X��������һ������ɫ���� D��m��n��p�������ʾ�Ϊ���ۻ�����
��ϰ��ϵ�д�
 ȫ�ŵ�����Ԫ�ƻ�ϵ�д�
ȫ�ŵ�����Ԫ�ƻ�ϵ�д�
�����Ŀ
18��2molA��2molB�����2L���ܱ������У��������·�Ӧ��2A��g��+3B��g��?2C��g��+zD��g����2s��A��ת����Ϊ50%�����v��D��=0.25mol•L-1•s-1�������ƶϲ���ȷ���ǣ�������
| A�� | z=2 | B�� | 2s�������ڵ�ѹǿ�dz�ʼ��$\frac{7}{8}$�� | ||
| C�� | 2sʱC���������Ϊ$\frac{2}{7}$ | D�� | 2s��B��Ũ��Ϊ0.5mol/L |


 3C��4D��Ӧ��
3C��4D��Ӧ�� ����ʾ�÷�Ӧ���������� �� ��
����ʾ�÷�Ӧ���������� �� �� H2(g)+I2(g)ƽ����ϵ�������С��ѹǿ�����ʹ��ɫ����
H2(g)+I2(g)ƽ����ϵ�������С��ѹǿ�����ʹ��ɫ����

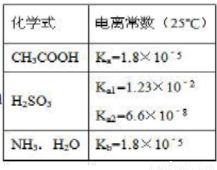
 OOH) =c (CH3COO-
OOH) =c (CH3COO- ) +2c (OH-)
) +2c (OH-)