��Ŀ����
13��25��ʱ�������йص������Һ��˵����ȷ���ǣ�������| A�� | ��Na2CO3��Һ��ˮϡ�ͺ�pH���Kw���� | |
| B�� | ����AgCl����ı�����Һ�м�����ˮ��c��Ag+����Ksp��AgCl�������� | |
| C�� | pH=4.75Ũ�Ⱦ�Ϊ0.1mol/L��CH3COOH��CH3COONa�Ļ����Һ�У�c��CH3COO-��+c��OH-����c��H+��+c��CH3COOH�� | |
| D�� | �ֱ���pH=2��pH=3��CH3COOH��Һ�к͵����ʵ�����NaOH������CH3COOH��Һ������ֱ�ΪVa��Vb����10Va��Vb |
���� A����Na2CO3��Һ��ˮϡ�ͣ�pH��С��
B������AgCl����ı�����Һ�м�����ˮ���ٽ�AgCl���ܽ⣬��Ϊ������Һ���¶Ȳ��䣬���ܶȻ����䣻
C��Ũ�Ⱦ�Ϊ0.1mol/L��CH3COOH��CH3COONa�Ļ����Һ����Һ�����ԣ�CH3COOH����̶ȴ���CH3COO-ˮ��̶ȣ���ϵ���غ��жϣ�
D������Ϊ������ʣ�Ũ��Խ����̶�ԽС��pHΪ2��3��CH3COOH��Һ�����ߵ���̶ȴ�
��� �⣺A����Na2CO3��Һ��ˮϡ�ͣ���Ȼ�ٽ�ˮ�⣬�����������pH��С����A����
B������AgCl����ı�����Һ�м�����ˮ���ٽ�AgCl���ܽ⣬��Ϊ������Һ����������Ũ�Ȳ��䣬���ܶȻ�ֻ���¶ȵ�Ӱ�죬�¶Ȳ��䣬���ܶȻ����䣬��B��ȷ��
C��Ũ�Ⱦ�Ϊ0.1mol/L��CH3COOH��CH3COONa�Ļ����Һ����Һ�����ԣ���Һ�д���c��CH3COO-��+c��OH-����c��H+��+c��Na+����CH3COOH����̶ȴ���CH3COO-ˮ��̶ȣ���c��Na+����c��CH3COOH������c��CH3COO-��+c��OH-����c��H+��+c��CH3COOH������C����
D��pH=2��pH=3��CH3COOH��pH=2�Ĵ������pH=3�Ĵ����10�����к͵����ʵ�����NaOH����pHΪ2�Ĵ���Ũ��Ϊx��PH=3�Ĵ���Ũ��Ϊy������Va��x=Vb��y����$\frac{{V}_{a}}{{V}_{b}}$=$\frac{y}{x}$��$\frac{1}{10}$����Vb��10Va����D����
��ѡB��
���� �����ۺϿ������ܵ���ʵ��ܽ�ƽ���Լ�������ʵĵ��룬Ϊ��Ƶ���㣬���ؿ���ѧ���ķ���������ע�� ��������ˮ�⡢����ʵĵ����Լ����ܵ���ʵ��ܽ�ƽ�����⣬�״���ΪC��ע�����غ�����ã��Ѷ��еȣ�

 �����������Ů��ͯ������ϵ�д�
�����������Ů��ͯ������ϵ�д�| A�� | 500mL0��l mol/LFeCl3��Һ�к�Fe3+0.05NA | |
| B�� | ���³�ѹ�£�2.24L��������NaOH��Һ�У�ת�Ƶ�����Ϊ0��lNA | |
| C�� | ���³�ѹ�£�28g��ϩ������C-H������ĿΪ4NA | |
| D�� | 0.5 molп��Ũ������ȫ��Ӧ����SO2��H2�Ļ�����壨�����������ܽ⣩������ķ�������С��0.5NA |
| A�� | NH4HCO3�����ֽ⣬���������� | |
| B�� | Na2CO3���м��ԣ�������θ���кͼ� | |
| C�� | SiO2�״������źţ����������ά | |
| D�� | Ca��ClO��2�ڿ����в��ȶ���������Ư��ֽ�� |
| Ԫ�� | Li | Be | B | C | O | F |
| Xֵ | 0.98 | 1.57 | 2.04 | 2.53 | 3.44 | 3.98 |
| Ԫ�� | Na | Al | Si | P | S | Cl |
| Xֵ | 0.93 | 1.61 | 1.90 | 2.19 | 2.58 | 3.16 |
��2���Ʋ�Xֵ��ԭ�Ӱ뾶�Ĺ�ϵ��ͬ���ڣ���ͬ���壩Ԫ�أ�xֵԽ��ԭ�Ӱ뾶ԽС��
��3��ij�л���ṹ��ʽΪ
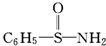 ����S��N�У�����Ϊ���õ��Ӷ�ƫ��˭������дԭ�����ƣ���
����S��N�У�����Ϊ���õ��Ӷ�ƫ��˭������дԭ�����ƣ�����4��������ɸ������ǵ��ɼ�����ԭ����ӦԪ�ص縺�ԵIJ�ֵ��X��1.7ʱ��һ��Ϊ���Ӽ�������X��1.7ʱ��һ��Ϊ���ۼ������ƶ�AlBr3�л�ѧ���������ǹ��ۼ�
��5��Ԥ��Ԫ�����ڱ��У�Xֵ��С��Ԫ��λ�õ������ڵڢ�A�壨������Ԫ�س��⣩��
| A�� | �ڼ���ȼú����������ʯ������Ч���ٶ���������ŷ��� | |
| B�� | ���������Դ��̫���ܡ���ϫ�ܡ����������� | |
| C�� | �����ŷ� SO2�� CO2 ���ᵼ��������γ� | |
| D�� | �������������ѹⴥý������������β���е� NO �� CO ת��Ϊ������ |
| A�� | SO2��NO2��������������ֱ����γ���������������������� | |
| B�� | ����[KAl��SO4��2•l2H2O]����ˮ���γɽ��壬��˿���������ˮ��ɱ������ | |
| C�� | ��������ֲ��������Ͻ����Ͻ���Ͼ���ǿ�ȴ������ᡢ����ʴ����ǿ���ŵ� | |
| D�� | �ճ������г�������ȥ�������ϵ����ۣ����ȵĴ�����Һϴ�Ӵ����ϵ����ۣ����ߵ�ԭ����ȫ��ͬ |

| A�� | ͼ��T1��T2 | |
| B�� | XZ������������pH��7 | |
| C�� | ����������������c��H+����c��OH+���TKW=1��10-13 | |
| D�� | M������������Ӧ����Һ���������Ӿ��ɴ������棺S2-��SO42-��Na+��Cl- |
| A�� | ��ⱥ��ʳ��ˮ����������22.4L����ʱ����·��ͨ���ĵ�����ĿΪ2NA | |
| B�� | 1L0.1mol•L-1̼������Һ�к��е���ԭ����Ϊ0.3NA | |
| C�� | �����£�56g��ƬͶ������ŨH2SO4������NA��SO2���� | |
| D�� | 0.1 mol��ϩ���Ҵ��Ļ������ȫȼ�������ĵ���������Ϊ0.3NA |
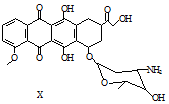
| A�� | ÿ��X�����к���5������̼ԭ�� | |
| B�� | һ�������£�X�����Ҵ�����������Ӧ | |
| C�� | һ�������£�X�ܷ�����ȥ��Ӧ | |
| D�� | X���������ᷴӦ��������NaOH��Һ��Ӧ |