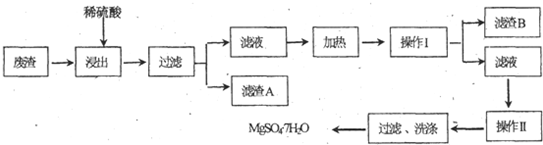
��Ŀ����
��ͼ�е�B��K�ֱ�����йط�Ӧ��һ�ַ�Ӧ������������A��C��F��K�ǹ��壻E�dz��������嵥�ʣ�I�Ǻ���ɫ����̬�������̬����A���������Ⱥ����ɵ�����������ͨ����ʯ��ֻʣ������B����ͨ��Ũ������ֻʣ������D�������ʼ��ת����ϵ����ͼ��ʾ��
��ش��������⣺

��1��B�Ļ�ѧʽΪ ��D�ĵ���ʽ ��
��2��д��ʵ���Ҽ���A�����к��е������ӵķ��� ��
��3��д��ʵ������ȡB�Ļ�ѧ����ʽ ��
��4����0.01mol Dͨ�� 1L 0.01mol/L F��Һ�У�������Һ����������Ũ���ɴ�С����˳��Ϊ ��
��5��д��N��ϡ��Һ����������۷�Ӧ�����ӷ���ʽ ��
��6�����������ͨ����ʯ�ҵõ�������B��ͨ��Ũ����õ�������D�����ʵ���֮����8��5���������ʵ����Ĺ�ϵ��ʾ�˹���A�����Ϊ ��
��ش��������⣺

��1��B�Ļ�ѧʽΪ
��2��д��ʵ���Ҽ���A�����к��е������ӵķ���
��3��д��ʵ������ȡB�Ļ�ѧ����ʽ
��4����0.01mol Dͨ�� 1L 0.01mol/L F��Һ�У�������Һ����������Ũ���ɴ�С����˳��Ϊ
��5��д��N��ϡ��Һ����������۷�Ӧ�����ӷ���ʽ
��6�����������ͨ����ʯ�ҵõ�������B��ͨ��Ũ����õ�������D�����ʵ���֮����8��5���������ʵ����Ĺ�ϵ��ʾ�˹���A�����Ϊ
���㣺������ƶ�,��������������ʼ���Ի�����Ӱ��
ר�⣺�ƶ���,����Ԫ��
������E�dz��������嵥�ʣ����Գ����ƶ�ΪO2��I�Ǻ���ɫ����̬�������ж�ΪNO2������ת����ϵ��֪HΪNO��GΪH2O��NΪHNO3��BΪNH3����̬����A���������Ⱥ����ɵ�����������ͨ����ʯ��ֻʣ������B��һ��ȷ��BΪNH3����ͨ��Ũ������ֻʣ������D��˵��DΪ������������ԭ�ԣ��Ʋ�DΪCO2�����ݷ�Ӧ��N��HNO3��+K��C��=D��CO2��+I��NO2��+G��H2O����CΪNa2O2��FΪ��Na2CO3��������A���ȷֽ����ɼ�������NH3����������CO2��֤��AΪ̼����Σ������ƶϳ������ʽ��з����ش����⣮
���
�⣺A��C��F��K�ǹ��壻E�dz��������嵥�ʣ�I�Ǻ���ɫ����̬�������̬����A���������Ⱥ����ɵ�����������ͨ����ʯ��ֻʣ������B����ͨ��Ũ������ֻʣ������D��E�dz��������嵥�ʣ����Գ����ƶ�ΪO2��I�Ǻ���ɫ����̬�������ж�ΪNO2������ת����ϵ��֪HΪNO��GΪH2O��NΪHNO3��BΪNH3������̬����A���������Ⱥ����ɵ�����������ͨ����ʯ��ֻʣ������B��һ��ȷ��BΪNH3����ͨ��Ũ������ֻʣ������D��˵��DΪ������������ԭ�ԣ��Ʋ�DΪCO2�����ݷ�Ӧ��N��HNO3��+K��C��=D��CO2��+I��NO2��+G��H2O����CΪNa2O2��FΪ��Na2CO3��������A���ȷֽ����ɼ�������NH3����������CO2��֤��AΪ̼����Σ�
��1�������ƶ�B�Ļ�ѧʽΪ��NH3��DΪCO2�ĵ���ʽ��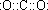 ���ʴ�Ϊ��NH3
���ʴ�Ϊ��NH3 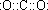 ��
��
��2��ʵ���Ҽ���A�����к��е���������NH4+�ķ�����ȡ����A���ʷ����Թ��У���������NaOH��ǿ����Һ�����ȣ����ܲ���ʹʪ��ĺ�ɫʯ����ֽ���������壬��֤������笠����ӣ�
�ʴ�Ϊ��ȡ����A���ʷ����Թ��У���������NaOH��ǿ����Һ�����ȣ����ܲ���ʹʪ��ĺ�ɫʯ����ֽ���������壬��֤������笠����ӣ�
��3��ʵ������ȡB��NH3���Ļ�ѧ����ʽΪ��Ca��OH��2+2NH4Cl
CaCl2+2H2O+2NH3����
�ʴ�Ϊ��Ca��OH��2+2NH4Cl
CaCl2+2H2O+2NH3����
��4����0.01mol D��CO2��ͨ�� 1L 0.01mol/L F��Na2CO3����Һ�У��������ʵ���������Ӧ����̼��������Һ��������Һ����������Ũ���ɴ�С����˳��c ��Na+����c��HCO3-����c ��OH-����c ��H+����c��CO32-����
�ʴ�Ϊ��c ��Na+����c��HCO3-����c ��OH-����c ��H+����c��CO32-����
��5��N��ϡ��ҺHNO3����������۷�Ӧ���������Σ���Ӧ�����ӷ���ʽΪ��3Fe+8H++2NO3-�T2NO��+3Fe2++4H2O��
�ʴ�Ϊ��3Fe+8H++2NO3-�T2NO��+3Fe2++4H2O��
��6���������ͨ����ʯ�ҵõ�������BΪNH3��ͨ��Ũ����õ�������D��CO2�������ʵ���֮����8��5�����ݻ�ѧ��Ӧ��ԭ���غ�õ�������̼����泥�笠����Ӻ�̼������������ʵ���֮��Ϊ1��1����Ϊ̼��泥�笠����Ӻ�̼����������ʵ���Ϊ2��1����������а����Ͷ�����̼���ʵ���֮��Ϊ8��5������1��1��2��1�䣬�����ж�AΪ̼��狀�̼����淋Ļ������ʵ���֮��ͨ��ƽ��ֵ������Ϊ3��2���������ʵ����Ĺ�ϵ��ʾ�˹���A�����ΪNH4HCO3�ͣ�NH4��2CO3�����ʵ���֮��Ϊ2��3��϶��ɣ�
�ʴ�Ϊ��NH4HCO3�ͣ�NH4��2CO3�����ʵ���֮��Ϊ2��3��϶��ɣ�
��1�������ƶ�B�Ļ�ѧʽΪ��NH3��DΪCO2�ĵ���ʽ��
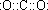 ���ʴ�Ϊ��NH3
���ʴ�Ϊ��NH3 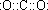 ��
����2��ʵ���Ҽ���A�����к��е���������NH4+�ķ�����ȡ����A���ʷ����Թ��У���������NaOH��ǿ����Һ�����ȣ����ܲ���ʹʪ��ĺ�ɫʯ����ֽ���������壬��֤������笠����ӣ�
�ʴ�Ϊ��ȡ����A���ʷ����Թ��У���������NaOH��ǿ����Һ�����ȣ����ܲ���ʹʪ��ĺ�ɫʯ����ֽ���������壬��֤������笠����ӣ�
��3��ʵ������ȡB��NH3���Ļ�ѧ����ʽΪ��Ca��OH��2+2NH4Cl
| ||
�ʴ�Ϊ��Ca��OH��2+2NH4Cl
| ||
��4����0.01mol D��CO2��ͨ�� 1L 0.01mol/L F��Na2CO3����Һ�У��������ʵ���������Ӧ����̼��������Һ��������Һ����������Ũ���ɴ�С����˳��c ��Na+����c��HCO3-����c ��OH-����c ��H+����c��CO32-����
�ʴ�Ϊ��c ��Na+����c��HCO3-����c ��OH-����c ��H+����c��CO32-����
��5��N��ϡ��ҺHNO3����������۷�Ӧ���������Σ���Ӧ�����ӷ���ʽΪ��3Fe+8H++2NO3-�T2NO��+3Fe2++4H2O��
�ʴ�Ϊ��3Fe+8H++2NO3-�T2NO��+3Fe2++4H2O��
��6���������ͨ����ʯ�ҵõ�������BΪNH3��ͨ��Ũ����õ�������D��CO2�������ʵ���֮����8��5�����ݻ�ѧ��Ӧ��ԭ���غ�õ�������̼����泥�笠����Ӻ�̼������������ʵ���֮��Ϊ1��1����Ϊ̼��泥�笠����Ӻ�̼����������ʵ���Ϊ2��1����������а����Ͷ�����̼���ʵ���֮��Ϊ8��5������1��1��2��1�䣬�����ж�AΪ̼��狀�̼����淋Ļ������ʵ���֮��ͨ��ƽ��ֵ������Ϊ3��2���������ʵ����Ĺ�ϵ��ʾ�˹���A�����ΪNH4HCO3�ͣ�NH4��2CO3�����ʵ���֮��Ϊ2��3��϶��ɣ�
�ʴ�Ϊ��NH4HCO3�ͣ�NH4��2CO3�����ʵ���֮��Ϊ2��3��϶��ɣ�
���������⿼��������ת����ϵ��Ӧ�ú��������ʵ�Ӧ�ã���Ҫ�������ʽ��д��笠����Ӽ��飬��ѧ����ʽ����д����Һ������Ũ�ȴ�С�ıȽϣ����ӷ���ʽ�IJ����жϺ���д��������ɵļ�������жϣ�

��ϰ��ϵ�д�
�����Ŀ
��NA��ʾ�����ӵ�����������˵����ȷ���ǣ�������
| A����0�棬ѹǿΪ101��105Paʱ��22.4L Cl2��HCl�Ļ�������к��е���ԭ������Ϊ3NA |
| B��0.5molI-������ʱʧȥ�ĵ�����Ϊ0.5NA |
| C��0.1L3 mol?L-1��NH4NO3��Һ�к��е�NH4+��ĿΪ0.3��6.02��1023 |
| D�����³�ѹ�£�48gO3������ԭ����Ϊ3NA |
��֪�����ԣ�Cl2��IO3-��Fe3+��I2���������Ӽ���Ľ��ۿɿ����ǣ�������
| A������Һ�м����������ˮ�ټӵ��ۣ���������ɫ��˵��û��I- |
| B����FeI2��Һ�еμ�������ˮʱ�����ӷ�ӦʽΪ��2I+Cl2=I2+2Cl |
| C����ij��Һ�м���ϡ���ᣮ������������ʹ����ʯ��ˮ����ǣ�˵������Һ����CO32-��HCO3- |
| D������Һ���ȼ���ϡHNO3���ټ�BaCl2��Һ���а�ɫ�������ɣ�˵����SO42- |
����˵����ȷ���ǣ�������
��ǿ�����һ�������ӻ�����������һ���ǹ��ۻ������ǿ�����һ����������ˮ�Ļ�����������һ����������ˮ�Ļ������ˮ�ѵ��룬��ˮ���������磬����ˮ��������ʡ������ڹ��ۻ�����ĵ����������״̬�²����磮
��ǿ�����һ�������ӻ�����������һ���ǹ��ۻ������ǿ�����һ����������ˮ�Ļ�����������һ����������ˮ�Ļ������ˮ�ѵ��룬��ˮ���������磬����ˮ��������ʡ������ڹ��ۻ�����ĵ����������״̬�²����磮
| A���٢� | B���ڢ� | C���ۢ� | D���٢� |
25��ʱ���й�����ĵ��볣�����±�������˵����ȷ���ǣ�������
| ���� | CH3COOH | HCN | H2CO3 |
| Ka | 1.8��10-5 | 4.9��10-10 | K1��4.3��10-7 K2��5.6��10-11 |
| A��������ʵ���Ũ����ҺpH��ϵ��pH ��NaCN����pH ��Na2CO3����pH ��CH3COONa�� |
| B��������ʵ���Ũ�ȵ�HCN��NaCN�����Һ�У�c ��HCN��+c��H+��=c ��OH-��+c ��CN-�� |
| C��a mol/LHCN��b mol/LNaOH��Һ�������Ϻ����õ���Һ�У���c��Na+����c ��CN-������aһ��С��b |
| D����Na2CO3���뵽HCN��Һ��ʱ�������·�Ӧ��Na2CO3+HCN=NaCN+NaHCO3 |
�����£����и���������ָ����Һ�п��ܴ���������ǣ�������
| A��FeCl3��Һ�У�Al3+��K+��MnO4-��SO42- |
| B�����ȳʻ�ɫ����Һ�У�K+��Br-��S2-��ClO- |
| C���������۲�����������Һ�У�Na+��NH4+��NO3-��Cl- |
| D��Kw/c��OH-��=0.1mol/L����Һ�У�Na+��K+��AlO2-��CO32- |
������Ǧ��������Ҫ��������Ϊ28%��ϡ���ᣮ����ʵ��������10g ��������Ϊ98%��Ũ���ᣨ�ܶ�Ϊ1.84g/cm3������28%��ϡ���ᣬ����������˵����ȷ���ǣ�ˮ���ܶȽ��ƿ���1g/cm3����������
| A����25mLˮ��������ʢ��5.4mLŨ�������Ͳ�У����ò��������Ͻ��� |
| B����10g98%��Ũ�������ձ�������ע��ʢ��25mLˮ���ձ��У����ò��������Ͻ��� |
| C��������������ȷ����ȡ25mLˮʱ���Ӷ�����������ϡ���������ʵ���������С��28% |
| D�����Ƹ���Һʱ������Ũ����մ������Ӧ����������������Һ�к� |