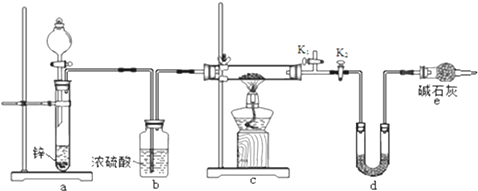
��Ŀ����
13�����ܼ����ֳ��ܻ�����2011����̨�屻�������ܻ���ʳƷ�Ѵ�961�6��1�������������������棬���ڱ�����������Ҳ��̪�����������ʣ�����ʳƷ�п���Υ�����ӵķ�ʳ�����ʺ������õ�ʳƷ���Ӽ����������й��̾����л��ϳ�ij���ܼ���·�ߣ���֪��������ڸ�����ص������£������ɱ��������Ȼ������磺��ͼ1��
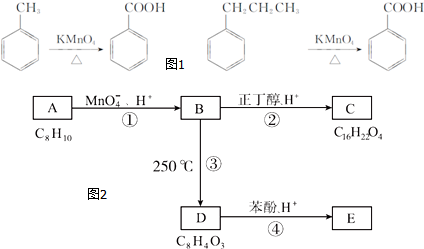
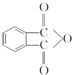
������A��E��ת����ϵ��ͼ2��ʾ����֪��A�Ƿ��㻯���ֻ������3��һ�廯���B�����ԣ�C�dz������ܼ���D���л��ϳɵ���Ҫ�м���ͳ��û�ѧ�Լ���DҲ��������ԭ�ϴ������õ�����E��һ�ֳ��õ�ָʾ����̪��
��1��A�Ļ�ѧ����Ϊ�ڶ��ױ���
��2��B�Ľṹ��ʽΪ
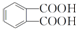 ��
����3����B����C�Ļ�ѧ����ʽΪ
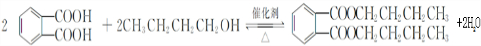 ���÷�Ӧ������Ϊȡ����Ӧ��
���÷�Ӧ������Ϊȡ����Ӧ����4��D�Ľṹ��ʽΪ
 ����D���ʵĺ˴Ź�������ͼ�У������2��壬�����֮��Ϊ1��1��
����D���ʵĺ˴Ź�������ͼ�У������2��壬�����֮��Ϊ1��1����5��A��ͬ���칹�������ڷ����廯�������3�֣�����һ�ȴ���������������
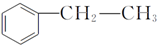 ��д�ṹ��ʽ����
��д�ṹ��ʽ����
���� A�ķ���ʽΪCH8H10�����ڷ��㻯���ֻ������3��һ�廯�����AΪ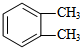 ��B�����ԣ���֪BΪ
��B�����ԣ���֪BΪ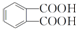 ��B������������������Ӧ����C�����C�ķ���ʽ��֪��C�Ľṹ��ʽΪ
��B������������������Ӧ����C�����C�ķ���ʽ��֪��C�Ľṹ��ʽΪ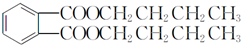 ��B���ȵõ�D�����D�ķ���ʽ��֪DΪ
��B���ȵõ�D�����D�ķ���ʽ��֪DΪ ��
��
��� �⣺A�ķ���ʽΪCH8H10�����ڷ��㻯���ֻ������3��һ�廯�����AΪ ��B�����ԣ���֪BΪ
��B�����ԣ���֪BΪ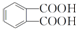 ��B������������������Ӧ����C�����C�ķ���ʽ��֪��C�Ľṹ��ʽΪ
��B������������������Ӧ����C�����C�ķ���ʽ��֪��C�Ľṹ��ʽΪ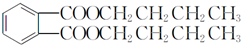 ��B���ȵõ�D�����D�ķ���ʽ��֪DΪ
��B���ȵõ�D�����D�ķ���ʽ��֪DΪ ��
��
��1��AΪ ������Ϊ�ڶ��ױ����ʴ�Ϊ���ڶ��ױ���
������Ϊ�ڶ��ױ����ʴ�Ϊ���ڶ��ױ���
��2��B�Ľṹ��ʽΪ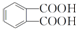 ���ʴ�Ϊ��
���ʴ�Ϊ��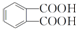 ��
��
��3����B����C�Ļ�ѧ����ʽΪ��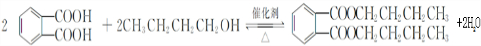 ���÷�Ӧ������Ϊȡ����Ӧ��
���÷�Ӧ������Ϊȡ����Ӧ��
�ʴ�Ϊ��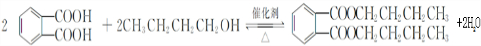 ��ȡ����
��ȡ����
��4��D�Ľṹ��ʽΪ ����D���ʵĺ˴Ź�������ͼ�У������2��壬�����֮��Ϊ 1��1��
����D���ʵĺ˴Ź�������ͼ�У������2��壬�����֮��Ϊ 1��1��
�ʴ�Ϊ�� ��2��1��1��
��2��1��1��
��5��A��ͬ���칹�������ڷ����廯������м���ױ����Զ��ױ����ұ�3�֣�����һ�ȴ���������������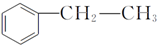 ��
��
�ʴ�Ϊ��3��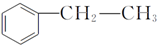 ��
��
���� ���⿼���л���ѧ�ƶϣ�ע������л������ʽ��������������չ�����������ת�����ѶȲ���

��ϰ��ϵ�д�
 �ƸԾ���Ȥζ����ϵ�д�
�ƸԾ���Ȥζ����ϵ�д� ����С����ҵ��ϵ�д�
����С����ҵ��ϵ�д�
�����Ŀ
3�����ʵ���Ũ����ͬ�����и������ʵ���Һ�У���ָ�����ӵ�Ũ������С�Ƚϣ����д�����ǣ�������
| A�� | c��PO43-����Na3PO4��Na2HPO4��NaH2PO4��H3PO4 | |
| B�� | c��CO32-������NH4��2CO3��Na2CO3��NaHCO3��NH4HCO3 | |
| C�� | c��NH4+������NH4��2SO4����NH4��2CO3��NH4HSO4��NH4Cl | |
| D�� | c��S2-����Na2S��NaHS��H2S |
4��2SO2��g��+O2��g���T2SO3��g���ķ�Ӧ�У���Ӧ1���Ӻ�SO3��Ũ��������0.9mol/L����O2��ʾ��ƽ������Ϊ��������
| A�� | 0.45mol/��L•min�� | B�� | 0.55mol/��L•min�� | C�� | 0.60mol/��L•min�� | D�� | 0.90mol/��L•min�� |
8���������ӷ���ʽ�У���ȷ���ǣ�������
| A�� | ����ͨ������У�CH3COOH+NH3�TCH3COO-+NH4+ | |
| B�� | ��̼����þ��Һ�мӹ���ʯ��ˮ��Mg2++2HCO3-+Ca2++2OH-�TCaCO3��+2H2O+MgCO3�� | |
| C�� | ����ʯ��ˮ��ϡ���ᷴӦ��Ca��OH��2+2H+�TCa2++2H2O | |
| D�� | ϡ�������ͭƬ�ϣ�Cu+2H+�TCu2++H2�� |
18�����ֶ�����Ԫ��W��X��Y��Z��ԭ�����������������ϱ�����Ϣ�ش��������⣮
��1��Z��Ԫ�����ڱ���λ�ڢ�A�壮
��2������Ԫ�ص�����������Ӧ��ˮ�����У���һ��������һ�������¾����������������ʷ�����ѧ��Ӧ����Ԫ����Na����Ԫ�ط��ţ���
��3�������п���Ϊ�Ƚ�X��Y������ǿ����������bc������ţ���
a����Ȼ���еĺ���
b����Ӧ�Ȼ���ˮ��Һ��pH
c��������ˮ��Ӧ�����׳̶�
d���������ᷴӦʱʧȥ�ĵ�����
�ڴ�ԭ�ӽṹ�ĽǶȽ���X�Ľ�����ǿ��Y��ԭ���Ӳ���ͬ���˵����Al��Na��ԭ�Ӱ뾶Na��Al������ԭ�Ӻ˶��������ӵ�������Na��Al��ʧ��������Na��Al��
��4��W��һ���⻯��HW3�������л��ϳɣ���������������ƣ������Ũ�Ⱦ���ȵ�HW3��X������������Ӧ��ˮ�������Һ��ϣ���Ӧ�Ļ�ѧ����ʽ��HN3+NaOH�TNaN3+H2O����Ϻ���Һ������Ũ���ɴ�С��˳����c��Na+����c��N3-����c��OH-����c��H+����
��5��Y���ʺ�Mg��ɵĻ������һ�����ԭ�ϣ�ij��ȤС�����������ʾ��ʵ�鷽�����ⶨ�������Y������������

��ȷ���������Y������������������abc������ţ���
a��m��n b��m��y c��n��y��
| W | X | Y | Z | |
| �ṹ������ | ����������Ӧ��ˮ����������̬�⻯�ﷴӦ�õ����ӻ����� | ��ɫ��Ӧ�ʻ�ɫ | ��ͬ��������Ԫ���γɵļ������У����Ӱ뾶��С | ��������������֮��Ϊ�� |
��2������Ԫ�ص�����������Ӧ��ˮ�����У���һ��������һ�������¾����������������ʷ�����ѧ��Ӧ����Ԫ����Na����Ԫ�ط��ţ���
��3�������п���Ϊ�Ƚ�X��Y������ǿ����������bc������ţ���
a����Ȼ���еĺ���
b����Ӧ�Ȼ���ˮ��Һ��pH
c��������ˮ��Ӧ�����׳̶�
d���������ᷴӦʱʧȥ�ĵ�����
�ڴ�ԭ�ӽṹ�ĽǶȽ���X�Ľ�����ǿ��Y��ԭ���Ӳ���ͬ���˵����Al��Na��ԭ�Ӱ뾶Na��Al������ԭ�Ӻ˶��������ӵ�������Na��Al��ʧ��������Na��Al��
��4��W��һ���⻯��HW3�������л��ϳɣ���������������ƣ������Ũ�Ⱦ���ȵ�HW3��X������������Ӧ��ˮ�������Һ��ϣ���Ӧ�Ļ�ѧ����ʽ��HN3+NaOH�TNaN3+H2O����Ϻ���Һ������Ũ���ɴ�С��˳����c��Na+����c��N3-����c��OH-����c��H+����
��5��Y���ʺ�Mg��ɵĻ������һ�����ԭ�ϣ�ij��ȤС�����������ʾ��ʵ�鷽�����ⶨ�������Y������������

��ȷ���������Y������������������abc������ţ���
a��m��n b��m��y c��n��y��
5����ѧ����ϡ�����ͻ���������أ������й�˵���д�����ǣ�������
| A�� | ú̿��������ʯ�;������ѻ����ѽ⻯���������ɻ�������Դ����Ҫ�Ļ���ԭ�� | |
| B�� | �������Ͷ����ص�Ǧ����ʹ��ʱ��ѹ���ȶ��������б��������͵��ȡ�������� | |
| C�� | �����Ʒ����Ҫ�ɷ��뽨������ɰ����ͬ | |
| D�� | ����10�ŷɴ�����̫���ܵ�ذ�ɽ�����ת��Ϊ���ܣ�����ת�����ϵ�����Ҳ�����Ʊ�����оƬ |
14���������й�����ʮ�˴�������ش���⣬��ͻ������̬�������ص�����ᷢչ����Ȼ����֮��ĺ�г��������Ϊ�в�������һ������ǣ�������
| A�� | �ƹ㡰��̼���á�����������������ŷ� | |
| B�� | ����̫���ܡ����ܺ����ܵ���Դ���滯ʯȼ�ϣ������ڽ�Լ��Դ���������� | |
| C�� | ���á���ɫ��ѧ�����գ�ʹԭ�Ͼ�����ת��Ϊ����Ҫ������ | |
| D�� | ��ͣ������ҵ��������ȾԴͷ |
 ������������һ�ֳ�Ӳ���ϣ����ɣ�2Mg3B2N4+3O2 $\frac{\underline{\;\;\;\;\;���£�����\;\;\;\;\;}}{���Ӽ�������}$6MgO+4BN+2N2�Ʊ���
������������һ�ֳ�Ӳ���ϣ����ɣ�2Mg3B2N4+3O2 $\frac{\underline{\;\;\;\;\;���£�����\;\;\;\;\;}}{���Ӽ�������}$6MgO+4BN+2N2�Ʊ���
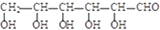 ��������������Ϊ
��������������Ϊ ��
��