��Ŀ����
7��Ϊ�˲ⶨһ����þ��Ϸ�ĩ����������������ijУ��ѧ��ȤС��ȡ����Ʒ8g���Ĵμ���ʢ��100gδ֪Ũ�ȵ�������Һ���ձ�����֪�ձ�����Ϊ50g���У���ַ�Ӧ����ձ����ձ������ʵ��������ݼ�¼���£�| ���� | 1 | 2 | 3 | 4 |
| ������Ʒ������/g | 2 | 2 | 2 | 2 |
| �ձ����ձ������ʵ�������/g | 151.9 | 153.8 | 157.7 |
��2������������Һ����������������14.7%��
��3����Ϸ�ĩ����������������70%��
���� ��1�����ݱ������ݷ������
��2����������������������������������������������
��3�����ݲμӷ�Ӧ�Ľ��������������������������û�ѧ����ʽ���н��
��� �⣺��1����ͼ�����ݿ�֪��ÿ����2g��Ʒ���������������Ϊ0.1g������150g+2g-151.9g=0.1g��151.9g+2g-153.8g=0.1g���ʵ����μ����Ϸ�ĩ���ձ����ձ�����Һ����������153.8g+2g-0.1g=155.7g�������μ����ĩ��δ��Ӧ���ձ����ձ������ʵ�������Ϊ155.7g+2g=157.7g�����Ե����μ����Ϸ�ĩ������ǡ�÷�Ӧ�ꣻ
��2��������������100g+50g+8g-157.7g=0.3g
��������Һ�����ʵ�����Ϊx
H2SO4����H2
98 2
x 0.3g
$\frac{98}{x}$=$\frac{2}{0.3g}$
x=14.7g
����������Һ���������������ǣ�$\frac{14.7g}{100g}$��100%=14.7%��
��3��������֪���μӷ�Ӧ�Ļ�Ϸ�ĩΪ6g����6g��ĩ����������Ϊm��þ������Ϊn��
Fe����H2����Mg����H2����
56 2 24 2
m $\frac{2m}{56}$ n $\frac{2n}{24}$
��m+n=6-------��
$\frac{2m}{56}$+$\frac{2n}{24}$=0.3---��
m=4.2g��n=1.8g
��Ϸ�ĩ���������������ǣ�$\frac{4.2g}{6g}$��100%=70%��
�ʴ�Ϊ����1��155.7��
��2��14.7%��
��3��70%��
���� ������Ҫ����ѧ���ж����ʼ�ķ�Ӧ��ϵ�Լ����û�ѧ����ʽ���м����������ѧ�������������Ӧ��ϵ����ȷ��д��ѧ����ʽ�����ܽ����

 A�ӽ��� ϵ�д�
A�ӽ��� ϵ�д� ȫ�Ų��Ծ�ϵ�д�
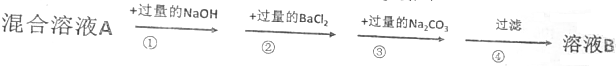
ȫ�Ų��Ծ�ϵ�д� ��һ����ɫ���壬���ܺ���CaCO3��Na2SO4��NaOH��BaCl2�е�һ�ֻ��֣�ȡ������ˮ���г������������˺�������еμ�������������������������������������Ĺ�ϵ��ͼ��ʾ���ɴ��ƶϰ�ɫ�����У�������
��һ����ɫ���壬���ܺ���CaCO3��Na2SO4��NaOH��BaCl2�е�һ�ֻ��֣�ȡ������ˮ���г������������˺�������еμ�������������������������������������Ĺ�ϵ��ͼ��ʾ���ɴ��ƶϰ�ɫ�����У�������| A�� | ����ֻ��CaCO3 | |
| B�� | ������һ����BaSO4��CaCO3 | |
| C�� | ��Һ�п�����CaCO3��NaOH��һ����Na2SO4��BaCl2 | |
| D�� | ��Һ��һ����Na2SO4��BaCl2��CaCO3��һ��û��NaOH |
 ��ͼ��ʾ��A��B��C��D�dz��л�ѧ���������ʣ�ͼ�С�������ʾת����ϵ�����ַ�Ӧ�������������ȥ��������A�Ǵ���ʯ����Ҫ�ɷ֣�B�����������壬C������θҺ�к��е��ᣮ��ش�
��ͼ��ʾ��A��B��C��D�dz��л�ѧ���������ʣ�ͼ�С�������ʾת����ϵ�����ַ�Ӧ�������������ȥ��������A�Ǵ���ʯ����Ҫ�ɷ֣�B�����������壬C������θҺ�к��е��ᣮ��ش�