��Ŀ����
12��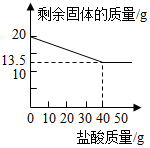 ij��ѧ��ȤС���ͬѧΪ�ⶨ�ٻƽ�ͭп�Ͻ����Ԫ�ص�������������ȡ20�˼ٻƽ������ձ��У���ijŨ�ȵ�ϡ����50�ˣ���5�μ��룬ÿ�γ�ַ�Ӧ��ȡ�����壬�����ˡ�����Ȳ�������أ�����ϡ����������ʣ�����������¼���£�
ij��ѧ��ȤС���ͬѧΪ�ⶨ�ٻƽ�ͭп�Ͻ����Ԫ�ص�������������ȡ20�˼ٻƽ������ձ��У���ijŨ�ȵ�ϡ����50�ˣ���5�μ��룬ÿ�γ�ַ�Ӧ��ȡ�����壬�����ˡ�����Ȳ�������أ�����ϡ����������ʣ�����������¼���£�| ʵ����� | ϡ�����������g�� | ʣ������������g���� |
| 1 | 10 | 18.375 |
| 2 | 10 | 16.750 |
| 3 | 10 | 15.125 |
| 4 | 10 | 13.500 |
| 5 | 10 | 13.500 |
��2����ͼ��֪��������ϡ������Ͻ��������Ϊ13��80ʱ������пǡ����ȫ�ܽ⣻
��3�����㵱ǡ����ȫ��Ӧʱ������Һ�����ʵ�����������
���� п��ϡ���ᷴӦ�����Ȼ�п��������ʣ������������Ϊͭ������������ͭ���������Լ���п�������������µ��������Լ������ɵ��Ȼ�п����������������������һ�����Լ���Ͻ���п������������ǡ�÷�Ӧʱ����������Һ�����ʵ�����������
��� �⣺��1���ɱ������ݿ�֪��ͭ������Ϊ��13.500g��
�Ͻ���п����������Ϊ��$\frac{13.500g}{20g}$��100%=67.5%��
�𣺺Ͻ���п����������Ϊ67.5%��
��2����ͼ�п�֪�������úϽ���ϡ�����������Ϊ����20g-13.500g����40g=13��80ʱ������пǡ����ȫ�ܽ⣮
���13��80��
��3���ɱ������ݿ�֪��п������Ϊ��20g-13.500g=6.5g��
�������Ȼ�п������Ϊx����������������Ϊy��
Zn+2HCl�TZnCl2+H2����
65 136 2
6.5g x y
$\frac{65}{6.5g}=\frac{136}{x}=\frac{2}{y}$��
x=13.6g��y=0.2g��
ǡ�÷�Ӧʱ����������Һ�����ʵ���������Ϊ��$\frac{13.6g}{6.5g+40g-0.2g}$��100%=29.4%��
��ǡ�÷�Ӧʱ����������Һ�����ʵ���������Ϊ29.4%��
���� ������Ҫ����ѧ�����û�ѧ����ʽ���м�����ƶϵ�������ͬʱ�����˷������ݵ�����������ʱҪע��淶�Ժ�ȷ�ԣ�

| A�� | BaCl2��Һ��ϡHNO3 | B�� | ��ɫʯ����Һ | ||
| C�� | NaOH��Һ | D�� | NaCl��Һ��ϡHNO3 |
| A�� | Cu | B�� | 2H | C�� | CO | D�� | N2 |
| A�� | NaCl | B�� | ��ʯ�� | C�� | �ռ� | D�� | ���� |