��Ŀ����
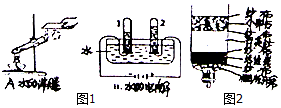
6����ͼ��ʾΪʵ�����г��������Ʊ�������������ռ�������ʵ��IJ�����������װʵ��װ��ʱ�����ظ�ѡ����������ij��ѧС���ͬѧ��������������и�̽��ʵ�飮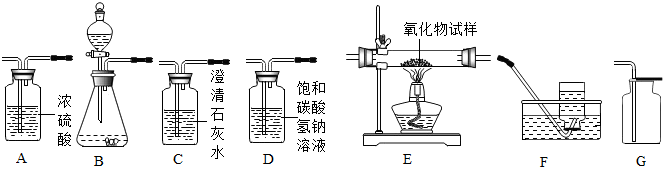
��1�����Թ���������ҺΪԭ�ϣ�������������������ʵ�������Ʊ����ռ������������
����ѡ����������˳��ΪB��A��G����д���������ĸ����
����װҩƷ֮ǰ�����װ��B�����Եľ�����������Ǽн���Ƥ�ܣ���Һ©����������Һ©���е�ˮ����һ���ֺ������£�˵��װ�ò�©����
����������ʱ������B�з�����Ӧ�Ļ�ѧ����ʽΪ2H2O2$\frac{\underline{\;MnO_2\;}}{\;}$2H2O+O2����
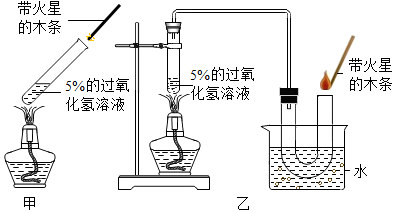
��2��С��ͬѧ������8������ͭ��ĩ����С�ĺ�һС����������ĩ��������һ��Сǿͬѧ�����û�������������̼��ˮ�����������ⶨ������������������������˳��Ϊ����������C��A1��E��A2��A3��
����֪��Fe2O3+3H2 $\frac{\underline{\;\;��\;\;}}{\;}$2Fe+3H2O��CuO+H2 $\frac{\underline{\;\;��\;\;}}{\;}$Cu+H2O������A1��A2��A3Ϊ����Ũ����ϴ��ƿ��
Сǿ��ͨ��������Ӧǰ��A2Ũ����ϴ��ƿ�������仯��������������Ʒ�к���Fe2O3��������ʵ����
������C�е��������ʯ��ˮ����ǣ�
�������ϴ��ƿA2����������7.2g��A3����û�䣬��������������������Ϊ16�ˣ�
������ϴ��ƿA2�������������������������������������������������������£���װ���в�����A1�����������ʵ��ֵ��ȽϽ�ƫ���á�ƫС����ƫ������Ӱ�족֮һ��д�հף���
������Eװ�ò������м��ٵ��������������������������������������������������£���װ���в�����A1�����������ʵ��ֵ��ȽϽ�����Ӱ�죨�á�ƫС����ƫ������Ӱ�족֮һ��д�հף���
���� ��1�����������ڶ������̴������·ֽ�����ˮ��������
��2��Ũ�����ܹ�����ˮ����������ʯ��ˮ�ܹ����鲢�����ն�����̼��
���ݷ�Ӧ�Ļ�ѧ����ʽ�����ṩ�����ݿ��Խ�����ط���ļ�����жϣ�
��� �⣺��1������ѡ����������˳��ΪB��A��G����ҺBװ����ȡ��������ҺAװ�ó�ȥˮ��������ҺGװ���ռ�������
���B��A��G��
����װҩƷ֮ǰ�����װ��B�����Եľ�����������ǣ��н���Ƥ�ܣ���Һ©����������Һ©���е�ˮ����һ���ֺ������£�˵��װ�ò�©����
����н���Ƥ�ܣ���Һ©����������Һ©���е�ˮ����һ���ֺ������£�˵��װ�ò�©����
����������ʱ������B�з�����Ӧ�Ļ�ѧ����ʽΪ��2H2O2$\frac{\underline{\;MnO_2\;}}{\;}$2H2O+O2����
���2H2O2$\frac{\underline{\;MnO_2\;}}{\;}$2H2O+O2����
��2��������C�е��������ʯ��ˮ����ǣ�
�������ʯ��ˮ����ǣ�
��������ͭ��������Ӧ����ˮ������Ϊx��
CuO+H2 $\frac{\underline{\;\;��\;\;}}{\;}$Cu+H2O��
80 18
8g x
$\frac{80}{8g}$=$\frac{18}{x}$��
x=1.8g��
��������������Ӧ����ˮ������Ϊ��7.2g-1.8g=5.4g��
������������Ϊy��
Fe2O3+3H2 $\frac{\underline{\;\;��\;\;}}{\;}$2Fe+3H2O��
160 54
y 5.4g
$\frac{160}{y}$=$\frac{54}{5.4g}$��
y=16g��
���16��
����װ���в�����A1�����²ⶨ��ˮ������ƫ�Ӷ����²�������ʵ��ֵ��ȽϽ�ƫ��
���ƫ��
������Eװ�ò������м��ٵ���������������������������ʱ���������м��ٵ�������Ϊ����������Ԫ�ص����������������������������£���װ���в�����A1����Ӱ������������Ԫ�ص����������������ʵ��ֵ��ȽϽ�����Ӱ�죮
�������Ӱ�죮
���� �������ʵ�飬��ѧ�ؽ���ʵ�顢����ʵ�飬�ǵó���ȷʵ����۵�ǰ�ᣬ���Ҫѧ�����ʵ�顢����ʵ�顢����ʵ�飬Ϊѧ�û�ѧ֪ʶ�춨������

| ѡ�� | ��Ҫ��������� | ���� |
| A | ������������������̼ | ��ȼ�ŵ�ľ�� |
| B | ����ء����ᱵ��̼��� | ����������ˮ |
| C | ϡ���ᡢ�Ȼ�����Һ������������Һ | �μ�̼������Һ |
| D | ���ۡ�ľ̿�ۡ�����ͭ��ĩ | ����������ϡ���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ʹ��ȼú����������ֹSO2��Ⱦ | |
| B�� | ����ʵʩ�̻����̣������ﳾ��Ⱦ | |
| C�� | �̾�����У�Ҫ���л��ﹲ���������ٿ�˽�ҳ� | |
| D�� | ����Ҫ���ദ�������ϴ���ɽ�������Ҫ��ʱ���� |
| A�� | N2 ��O2��--�����建��ͨ�����ȵ�ͭ�� | |
| B�� | FeSO4��Һ��CuSO4��Һ��--������������ۣ����� | |
| C�� | NaCl��Һ��Na2SO4��--���������BaCl2��Һ������ | |
| D�� | NaCl���壨��ɳ��--��ˮ�ܽ⡢���ˡ����� |
| ���ʣ�������Ϊ���ʣ� | ���ӷ��� | |
| A | NaCl����ɳ�� | �ܽ⡢���ˡ����� |
| B | FeCl2��CuCl2�� | ������������ |
| C | ��ʯ������������ʯ�� | ����������ˮ |
| D | CO2��CO�� | ͨ�����ʯ��ˮ |
| A�� | A | B�� | B | C�� | C | D�� | D |
 |  |  |  |
| A������ȼ�����ɶ�����̼��ˮ | B������һ������������������ȼ�� | C�������Ż��Ⱥ��� | D��������̼���ܶȱȿ�����ȼ��Ҳ��֧��ȼ�� |
| A�� | A�� | B�� | B�� | C�� | C�� | D�� | D�� |