��Ŀ����
17�����꣬�ҹ��������ܶ�����������˲�ͬ�̶ȵĺ��飬ȫ�������Ž�һ�£���ͬ����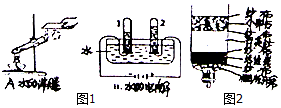
��1����Щ��ׯ���ȡ�õ���ˮ���������ˮ��Ӳˮ������ˮ����ѡ�õ������Ƿ���ˮ��ʹ��Ӳˮ���������������������鷳�����˷ѷ�������һ����
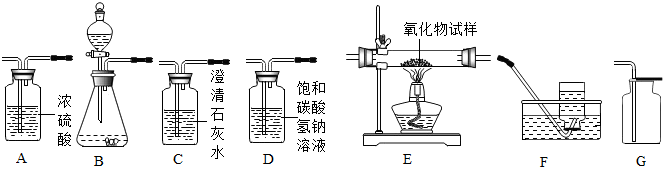
��2��ͼ1ʵ��B��û�з����ı��������ԭ�Ӻ���ԭ�ӣ��Թ�2�еõ���������������
��3����Щ����ȡ���ǵĿ�ˮ��������ˮ��С��ͬѧ˵�������ù��˵ķ�����ȥˮ�еIJ��������ʣ����ù��˵ķ�����ȥ���ʣ����˲��������õ��IJ����������ձ�����������©����С��ͬѧ˵���Խ����ǵĿ�ˮ�ü���ˮ������ͼ2��ʾ�����н���������С��ʯ��ʯӢɰ�������ǹ��ˣ�Ϊ�������ĵ���ˮ��Դ�������������һ����Լ��ˮ�Ľ���ϴ��ˮ�ϵأ�
���� �������е�ˮ�ľ��������ˮ�Լ���Լ��ˮ��֪ʶ���з�����ɣ�
��� �⣺��1������Ӳˮ����ˮʹ�õ��Ƿ���ˮ��Ӳˮϴ�·����ɾ�������ɷ������˷ѣ��������ˮ���˷ѷ�����
��2����ѧ�仯�е���С������ԭ�ӣ����ˮʵ������С��������ԭ�Ӻ���ԭ�ӣ��Թ�2���Դ�������������õ����������������ԭ�Ӻ���ԭ�ӣ�������
��3�����˲��������õ��IJ����������ձ�����������©��������ˮ����С��ʯ��ʯӢɰ�������ǹ��ˣ�ϴ��ˮ�ϵ�����Լ��ˮ�����ã����©�������ˣ�ϴ��ˮ�ϵأ�
���� ����ˮ�ľ����Լ���Լ��ˮ��֪ʶ����ȷ�����ؼ���

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
8��CO2����Ҫ����Դ������Ϊԭ�Ͽɻ�������������ʣ�����˵��������ǣ�������


| A�� | ��Ӧ����̫����ת��Ϊ��ѧ�� | |
| B�� | ��Ӧ�ڵ�ʵ�������ڻ��⡰��ɫ��Ⱦ�� | |
| C�� | ��Ӧ���ǻ��Ϸ�Ӧ | |
| D�� | ��Ӧ�ܻ�ò�Ʒ���ʣ��Һ�У�����һ����NaHCO3 |
12��������ͼ�������ӵĽṹʾ��ͼ�������Ϣ����ȷ���ǣ�������

| A�� | ���DZ�ʾ����Ԫ�� | B�� | �٢��ǽ���Ԫ�� | ||
| C�� | �٢ܢݵĻ�ѧ���ʶ��ȶ� | D�� | ���ڻ�ѧ��Ӧ���õ��� |
2�����ش���ȫ�������������������ؾ���ˮ��������������ӻ�ѧ���ӽ�����Ϊ���в����ϸ�������ǣ�������
| A�� | �����̻�����ȼ�� | B�� | ��Ҫʱ��˽�ҳ����� | ||
| C�� | �������ദ�� | D�� | ������ˮֱ�ź��� |
9�� ��ͼΪij�����ܽ�����¶ȱ仯�����ߣ��ù������Һ������ʱ�����ᾧˮ��M��N����ֱ��ʾ�ù����γɵ�������Һ�ڲ�ͬ�¶�ʱ��Ũ�ȣ��������ı�ʱ����Һ�µ�״̬��ͼ�ж�Ӧ�ĵ��λ�ÿ���Ҳ��֮�仯�������жϲ���ȷ���ǣ�������
��ͼΪij�����ܽ�����¶ȱ仯�����ߣ��ù������Һ������ʱ�����ᾧˮ��M��N����ֱ��ʾ�ù����γɵ�������Һ�ڲ�ͬ�¶�ʱ��Ũ�ȣ��������ı�ʱ����Һ�µ�״̬��ͼ�ж�Ӧ�ĵ��λ�ÿ���Ҳ��֮�仯�������жϲ���ȷ���ǣ�������
 ��ͼΪij�����ܽ�����¶ȱ仯�����ߣ��ù������Һ������ʱ�����ᾧˮ��M��N����ֱ��ʾ�ù����γɵ�������Һ�ڲ�ͬ�¶�ʱ��Ũ�ȣ��������ı�ʱ����Һ�µ�״̬��ͼ�ж�Ӧ�ĵ��λ�ÿ���Ҳ��֮�仯�������жϲ���ȷ���ǣ�������
��ͼΪij�����ܽ�����¶ȱ仯�����ߣ��ù������Һ������ʱ�����ᾧˮ��M��N����ֱ��ʾ�ù����γɵ�������Һ�ڲ�ͬ�¶�ʱ��Ũ�ȣ��������ı�ʱ����Һ�µ�״̬��ͼ�ж�Ӧ�ĵ��λ�ÿ���Ҳ��֮�仯�������жϲ���ȷ���ǣ�������| A�� | ������10���M��N�������ƽ�� | |
| B�� | ��ˮϡ�ͣ������¶ȶ����䣩ʱ��M��N��������� | |
| C�� | ������10���M���������������ƣ�N������ƽ�� | |
| D�� | �����ܼ��������¶ȶ����䣩ʱ������M�㲻����N����ƽ�������ߣ����������ܼ���M��N�㶼���� |
7�����в�����������Ȼ�л��߷��Ӳ��ϵ��ǣ�������
| A�� | ��˿ | B�� | ���� | C�� | ���� | D�� | ���� |
 ��ͼ��ѧ��Ӧ����Һ��ɫ�仯�����ˡ�ħ�����磬������ѧ������ش�
��ͼ��ѧ��Ӧ����Һ��ɫ�仯�����ˡ�ħ�����磬������ѧ������ش�