��Ŀ����
2��ͨ��ѧϰ������ʣ�ijУ��ѧ��ȤС���ͬѧ��ʵ���ҵġ����������Ƿ�����Լ����ʵij̶ȡ�չ����̽���������һͬ���룮��1��������⣺���������Ƿ���ʣ�
��2����������裺ͬѧ�Ǿ���������Ϊ��Ʒ�������ֿ��������û�б��ʣ����ֱ��ʣ���ȫ���ʣ�
����������Ʊ��ʣ����ʵĻ�ѧ���̶�Ϊ��Ca��OH��2+CO2�TCaCO3��+H2O��
��3��ʵ��̽����������գ�
| ʵ�鲽�輰���� | ʵ������ | ʵ����� |
| ��ȡ�����Թ��У�������������ˮ������ȡ�ϲ���Һ��������ɫ��̪��Һ�� �ڵ�ȥ�ϲ���Һ�������Թ���ע��ϡ���� | ����ɫ��̪��Һ��죻 �������ݲ��� | ���ֱ��� |
| ����ɫ��̪��Һ����죻 �������ݲ��� | ȫ������ | |
| ����ɫ��̪��Һ��죻 ��û�����ݲ��� | û�б��� |
��С��ͬѧ��������ʵ�鷽������ʵ�飬ȷ������Ʒ�Ѳ��ֱ��ʣ���д��ʵ���з�Ӧ�漰�Ļ�ѧ����ʽ��CaCO3+2HCl�TCaCl2+H2O+CO2��
�����������׳���ʯ�ң�ũҵ�ϳ������������������ȣ�
��5��ij����������������������Һ�к�һ����ʯ�Ͳ�Ʒ�еIJ������������ԣ�������5%������������Һ80g����һ����ʯ�Ͳ�Ʒ�к�H2SO4�������Ƕ��٣�
���� ��2�������������Ʊ������������̼��Ӧ����̼��ƺ�ˮд����صķ���ʽ��
��3�������������Ƶı��ʳ̶ȷ�������û�б��ʣ�ֻ�����������ƣ��ӷ�̪��Һ�ῴ����̪��Һ��ɺ�ɫ����ϡ����û�����ݲ�����
�ڲ��ֱ��ʣ������������ƺ�̼��ƣ��ӷ�̪��Һ�ῴ����̪��Һ��ɺ�ɫ����ϡ���������ݲ�����
��ȫ�����ʣ�ֻ����̼��ƣ��ӷ�̪��Һ���ῴ����̪��Һ��ɺ�ɫ����ϡ���������ݲ�����
��4���ٴ�ʵ����ۣ������ֱ��ʡ�����֪�����к���̼��ƺ���������ȥ������
�ڴ����������׳���ʯ�ң�ũҵ�ϳ�����������������������ũҩ������Һ��ȥ������
��5���������⣬����������Һ�����ᷴӦ���������ƺ�ˮ��������5%������������Һ80g�������������=��Һ���������ʵ�������������Ӧ�Ļ�ѧ����ʽ���з�����ɣ�
��� �⣺��2���������Ʊ����������̼��Ӧ����̼��ƺ�ˮ���ʷ�Ӧ�ķ���ʽΪ��Ca��OH��2+CO2�TCaCO3��+H2O��
��3����ʵ����ۣ������ֱ��ʡ�����֪�����к���̼��ƺ��������ƣ�����ȡ�����Թ��У�������������ˮ�����â�ȡ�ϲ���Һ��������ɫ��̪��Һ������������Һ�ʼ��ԣ���ʹ��ɫ��̪��Һ��죻�ڵ�ȥ�ϲ���Һ�������Թ���ע��ϡ���ᣬϡ�����̼��ƣ����ڼ�������������ˮ����������ȫ���ܽ���ˮ�У���Ӧ���ɶ�����̼���壻
��ʵ����ۣ���ȫ�����ʡ�����֪�����к���̼��ƣ�����ȡ�����Թ��У�������������ˮ�����â�ȡ�ϲ���Һ��������ɫ��̪��Һ������û������������Һ��������ɫ��̪��Һ����ɫ���ڵ�ȥ�ϲ���Һ�������Թ���ע��ϡ���ᣬϡ�����̼��Ʒ�Ӧ���ɶ�����̼���壻
��ʵ����ۣ���û�б��ʡ�����֪�����к���ֻ���������ƣ�����ȡ�����Թ��У�������������ˮ�����â�ȡ�ϲ���Һ��������ɫ��̪��Һ������������Һ�ʼ��ԣ���ʹ��ɫ��̪��Һ��죻�ڵ�ȥ�ϲ���Һ�������Թ���ע��ϡ���ᣬ����û��̼��ƣ����Բ��ᷴӦ���ɶ�����̼���壻
��������ݲ����������ݲ�������ɫ��̪��Һ��죻
��4������ʵ����ۣ������ֱ��ʡ�����֪�����к���̼��ƺ��������ƣ�����ȡ�����Թ��У�������������ˮ�����â�ȡ�ϲ���Һ��������ɫ��̪��Һ������������Һ�ʼ��ԣ���ʹ��ɫ��̪��Һ��죻�ڵ�ȥ�ϲ���Һ�������Թ���ע��ϡ���ᣬϡ�����̼��ƣ����ڼ�������������ˮ����������ȫ���ܽ���ˮ�У���Ӧ���ɶ�����̼����ˮ���Ȼ��ƣ��䷴Ӧ�Ļ�ѧ����ʽΪ��CaCO3+2HCl�TCaCl2+H2O+CO2�����ʴ�Ϊ��CaCO3+2HCl�TCaCl2+H2O+CO2����
�����������׳���ʯ�ң�ũҵ�ϳ�����������������������ũҩ������Һ�ȣ��ʴ�Ϊ���������������ȣ�
��5���⣺��һ����ʯ�Ͳ�Ʒ�к�H2SO4������Ϊx��
2NaOH+H2SO4�TNa2SO4+2H2O
80 98
80g��5% x
$\frac{80}{80g��5%}$=$\frac{98}{x}$ x=4.9g
��һ����ʯ�Ͳ�Ʒ�к�H2SO4��������4.9g��
���� �����ۺ��Խ�ǿ������ѧ�����й��������Ƶ���;�����ʵ�ԭ���ʺ�����������ʵ�������Ӧ�ü�������ý��з������⡢��������������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| ѡ�� | ʵ��Ŀ�� | ʵ����� |
| A | �ӿ����л�ȡ�����ĵ��� | ����ȼ�ճ�ȥ�����е����� |
| B | ����Ӳˮ����ˮ | �۲���ɫ��μӷ���ˮ |
| C | ��ȥ��������������Һ�е�̼������Һ | ��������ϡ���� |
| D | ��������狀��Ȼ��ƹ��� | ��������ˮ�ܽ⣬����ǰ���¶ȱ仯 |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | �� | B�� | �� | C�� | �� | D�� | п |
| A�� |  �㵹Һ�� | B�� | 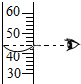 ��ȡ��ֵ | C�� |  ����Һ�� | D�� |  ��������� |
��1��д�����кͷ�Ӧ�Ļ�ѧ����ʽCa��OH��2+2HCl�TCaCl2+2H2O��
��2��̽���ձ�����Һ�����ʵijɷ֣�
��������⡿���ձ��ڵ���Һ��������ʲô��
�����в��롿�����ʿ���ֻ��CaCl2
�����ʿ�����CaCl2��Ca��OH��2
�����ʿ�����CaCl2��HCl
��ʵ��̽����С��ͬѧΪ��̽����Һ�����ʵijɷ֣�����������ʵ��̽����
| ʵ�鲽�� | ʵ������ | ʵ����� |
| ȡ������Ӧ�����Һ���Թ��У����Թ��еμӼ�����ɫ��̪��Һ���� | ��Һ����ɫ | ����ڲ���ȷ |
| �������ȷ |
| A�� | ������Һ��ʹ��̪��Һ��죬������ʹ��̪��Һ������Һһ���ʼ��� | |
| B�� | �����Ǵ���ɵ����ӣ����Դ���ɵ�����һ�������� | |
| C�� | �л��ﶼ����̼Ԫ�أ����Ժ�̼Ԫ�صĻ�����һ�����л��� | |
| D�� | ������ͬ��Ԫ����ɣ�������ͬ��Ԫ����ɵ�����һ���ǵ��� |
