��Ŀ����
15��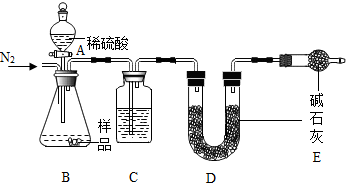 ��֪NaHCO3��270��ʱ��ȫ�ֽ�Ϊ̼���ơ�ˮ�Ͷ�����̼����Na2CO3���Ȳ��ֽ⣮����ij������������һ��
��֪NaHCO3��270��ʱ��ȫ�ֽ�Ϊ̼���ơ�ˮ�Ͷ�����̼����Na2CO3���Ȳ��ֽ⣮����ij������������һ��NaHCO3��Ʒ�л���������Na2CO3��ij��ȤС��ͬѧΪ�˲ⶨ�ò�Ʒ��NaHCO3�������������������������ʵ�鷽��
����һ����Ʒ$\stackrel{ϡ����}{��}$�ⶨ����CO2��������
��1����ʵ�������ͼ��ʾ��װ�ã�C��ʢ�ŵ�������Ũ���ᣮ
��2�����ѷ�Һ©���е�ϡ�����Ϊϡ���ᣬ������������ȷ������£����ܣ���ܡ����ܡ���ȷ�ⶨ��Ʒ��NaHCO3������������
����������Ʒ$\stackrel{����}{��}$�ⶨʣ�����������
��������������£�
��ȡһֻ�ྻ��������Ƶ�����Ϊ21.2�ˣ�Ƚ�����м�����Ʒ���Ƶ�������Ϊ41.2�ˣ�
�ڼ���ʢ����Ʒ��������
�۽����������ȴ������������ʣ������������
�ܶ���ظ�����ں͢������أ��Ƶ�������ʣ������������Ϊ35.0�ˣ�
��3��ʵ��ʱ��Ҫ�ظ������ȡ���ȴ��������������Σ���Ŀ����ʹ̼��������ȫ��Ӧ��
��4���������⣬������Ʒ��NaHCO3������������д��������̣�����֪��2NaHCO3$\frac{\underline{\;\;��\;\;}}{\;}$Na2CO3+H2O+CO2����
���� ��1�����ݼ�ʯ�һ�����ˮ�ֺͶ�����̼���з�����
��2�����ݼ�ʯ���Ǽ��Ը������������лӷ��Խ��з�����
��3������ʵ��ʱ��Ҫ�ظ������ȡ���ȴ��������������Σ�����ʹ̼��������ȫ�ֽ���з�����
��4������ʵ������м��ٵ�������ˮ�Ͷ�����̼���������з�����
��� �⣺��1����ʯ�һ�����ˮ�ֺͶ�����̼��ʵ�����ü�ʯ�����ն�����̼֮ǰ����Ҫ��������C��ʢ�ŵ�������Ũ���
��2����ʯ���Ǽ��Ը������������лӷ��ԣ���������������Ӱ�죬���Բ���ȷ�ⶨ��Ʒ��NaHCO3������������
��3��ʵ��ʱ��Ҫ�ظ������ȡ���ȴ��������������Σ�����ʹ̼��������ȫ�ֽ⣻
��4��ʵ������м��ٵ�������ˮ�Ͷ�����̼�������������������Ϊ��41.2g-35g=6.2g��
����Ʒ�к���̼������Ϊx��
2NaHCO3$\frac{\underline{\;\;��\;\;}}{\;}$Na2CO3+H2O+CO2�� �������
168 62
x 6.2g
$\frac{168}{x}$=$\frac{62}{6.2g}$
x=16.8g
��Ʒ��̼������Ϊ��41.2g-21.2g=20g
������Ʒ��NaHCO3����������Ϊ��$\frac{16.8g}{20g}$��100%=84%��
�ʴ�Ϊ����1��Ũ�����2�����ܣ���3��ʹ̼��������ȫ��Ӧ����4��84%��
���� ���⿼����̼���Ƶ������Լ��йصļ��㣬��ɴ��⣬�����������е�֪ʶ���У�

| A�� | ���� | B�� | ֲ���� | C�� | ���� | D�� | ʳ�� |
| A�� | ���ӿ����ٷ� | B�� | ����֮���м�� | ||
| C�� | �����ڲ����˶� | D�� | ���ӿ��Թ������� |
| A�� | ��ɫ����ˮ����ͭ��ˮ����ɫ������ΪCuSO4��H2Oת��ΪCuSO4•5H2O | |
| B�� | ���ü���Ȯ�ܸ�����ζ���ֶ�Ʒ������Ϊ�����ڲ����˶� | |
| C�� | 5mL�ƾ���5mLˮ��Ϻ����С��10mL������Ϊ��Ϲ����з��ӱ�С�� | |
| D�� | ��ͬ����Ļ�ѧ����������ͬ������������ɵ������Ӳ�ͬ�й� |

| A�� | Ԫ��A��B��C�ֱ��ʾ̼���⡢�� | |
| B�� | �����ʻ�ѧʽ�ɱ�ʾΪCH6O2 | |
| C�� | ��������Է�������Ϊ105 | |
| D�� | ������̼���⡢������ԭ�Ӹ�����Ϊ2��6��1 |

��ͼ������d�������ǣ�����©����
��Ҫ��ȡ�϶��O2����̽�������ʣ�Ҫ����ȡ�����п������ӷ�Ӧ�����װ���巢��װ����Ҫ��������adf������ţ���ͬ������д���÷�Ӧ�Ļ�ѧ����ʽ2H2O2$\frac{\underline{\;MnO_2\;}}{\;}$2H2O+O2�����ռ�����Ӧ���������ſ��������������ռ������Ǹ����������ܶȱȿ������ܶȴ����ʣ�
���ᴿ����ʱ��������Ҫѡ�����������е�eh������ţ�������֮�ȱ�ٵ���������ֽ��©�����������������ƣ���
��2��Ϊ̽������X����ɣ�ij��ȤС�������ͼ��ʾʵ�飨�̶�װ��δ��������
���������ߡ�
�ټ��������£�����X��������ͭ��Ӧ����ͭ��ˮ�͵�����
�ڼ�ʯ��Ϊ�����������ƺ��������ƵĻ�����������X��Ӧ����ˮ�Ȼ��ƿ���������X��
�۱�ʵ�������£������ܶ�Ϊ1.15g•L-1��װ���ڿ����е�ˮ�������̼��������ݲⶨ��Ӱ��ɺ��Բ��ƣ���ʵ�鲽�衿
�����������������װ�������ԣ�
��ȡһ��������ͭ��ȷ�Ƶ�������Ϊ2.40g��
�۰�ͼ��ʾװ��ҩƷ������B��C���зֱ�װ�����������ʯ�Һ�������ˮ�Ȼ��ƣ����Ⲣ��¼�������ݢ�������
������A��B��Cװ�ã�������X����ͨ��һ��ʱ���������Dװ�ã�������ʼ���ȣ���Aװ��������ͭ��Ӧ��ȫ��ֹͣ���ȣ�����ͨ������X����������ȴ���ٴβ�������¼������ݢ�������
��ʵ��װ�á�

��������ݡ�
| ������Ŀ/���ݱ�� | �� | �� |
| �����ܣ���ҩƷ��������/g | 52.40 | 51.92 |
| Bװ�ã���ҩƷ��������/g | 102.00 | 102.54 |
| ���������/mL | / | 243.5 |
��B��װ������������ˮ��ԭ��ΪCaO+H2O=Ca��OH��2���û�ѧ����ʽ��ʾ����
��ʵ������У����۲쵽Dװ���������ܵ�Һ�治���½���˵������ͭ�ѷ�Ӧ��ȫ��ֹͣ���ȣ�����ͨ������X����������ȴ��Ŀ���Ƿ�ֹ������������ɵ�ͭ�����������
�۷���ʵ����������ݣ�����ˮ������Ϊ0.54g������ͭ����Ԫ�ص�����Ϊ0.48g���ɴ���֪������X��һ��û�У���С���û�С�����Ԫ�أ�
��ͨ�������Ƶ�������X�Ļ�ѧʽΪNH3������X��ԭ����ͭ�Ļ�ѧ����ʽΪ��2NH3+3CuO$\frac{\underline{\;\;��\;\;}}{\;}$3Cu+3H2O+N2��
 ��һ������AgNO3��Cu��NO3��2�Ļ����Һ�м��������Zn�ۣ���Һ�����淴Ӧʱ��仯�������ͼ��ʾ������˵����ȷ���ǣ�������
��һ������AgNO3��Cu��NO3��2�Ļ����Һ�м��������Zn�ۣ���Һ�����淴Ӧʱ��仯�������ͼ��ʾ������˵����ȷ���ǣ�������| A�� | ȡa����Һ���μ�ϡ���ᣬ�ް�ɫ���� | |
| B�� | ͼ����b-c���������ӵ�ԭ����Zn��Cu��NO3��2��Һ��Ӧ | |
| C�� | b��ʱ��Һ�н���������Zn2+��Cu2+������������Zn��Cu��Ag | |
| D�� | c���d���������ͬ��������������Ҳ��ͬ |
 ͼ�е�A��H�dz��л�ѧ�����Ļ�����ҷֱ�����H��O��S��Na��Ca��Cu�е����ֻ�����Ԫ����ɵģ�����B��E�������E������Ԫ������֮��Ϊ2��3��C��F��HΪ��ͬ���Ļ����F������ˮ���ų��������ȣ�G����Һ����ɫ��ͼ�С�--����ʾ���˵����ʼ��ܷ�����ѧ��Ӧ����������ʾ���ʼ����ת����ϵ�����ַ�Ӧ��������Ӧ��������ȥ��
ͼ�е�A��H�dz��л�ѧ�����Ļ�����ҷֱ�����H��O��S��Na��Ca��Cu�е����ֻ�����Ԫ����ɵģ�����B��E�������E������Ԫ������֮��Ϊ2��3��C��F��HΪ��ͬ���Ļ����F������ˮ���ų��������ȣ�G����Һ����ɫ��ͼ�С�--����ʾ���˵����ʼ��ܷ�����ѧ��Ӧ����������ʾ���ʼ����ת����ϵ�����ַ�Ӧ��������Ӧ��������ȥ��