��Ŀ����
9��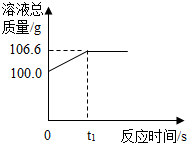 Ϊ�ⶨij������ʯ��������������������С��������ͬѧ��������һ����̼��10g������ʯ��Ʒ��ַ�Ӧ�����ʲ����뷴Ӧ�����������ɵ�������һ����������������Һ��ȫ���գ�����Һ�������뷴Ӧʱ��ı仯��ϵ��ͼ��
Ϊ�ⶨij������ʯ��������������������С��������ͬѧ��������һ����̼��10g������ʯ��Ʒ��ַ�Ӧ�����ʲ����뷴Ӧ�����������ɵ�������һ����������������Һ��ȫ���գ�����Һ�������뷴Ӧʱ��ı仯��ϵ��ͼ����1��������Ӧ����������̼������Ϊ6.6g��
��2���ó�����ʯ����Ԫ�ص���������Ϊ80%��
��3���������������Һǡ����ȫ��Ӧ������������������Ӧ�����Լ�����������������Һ����������������
���� ��������һ����̼�ڸ��������·�Ӧ�������Ͷ�����̼��������̼���������Ʒ�Ӧ����̼���ƺ�ˮ������������Һ���ӵ�������Ϊ��Ӧ���ɶ�����̼�����������ݶ�����̼���������Լ������������������Ӷ����Լ���ó�����ʯ����Ԫ�ص�����������
���ݶ�����̼���������Լ����������Ƶ���������һ�����Լ�����������������Һ����������������
��� �⣺��1����ͼ�����ݿ�֪�����ɶ�����̼������Ϊ��106.6g-100.0g=6.6g��
���6.6��
��2����������������Ϊx��
Fe2O3+3CO$\frac{\underline{\;����\;}}{\;}$2Fe+3CO2��
160 132
x 6.6g
$\frac{160}{x}$=$\frac{132}{6.6g}$��
x=8g��
�ó�����ʯ����Ԫ�ص���������Ϊ��$\frac{8g}{10g}$��100%=80%��
���80%��
��3����������������Ϊy��
2NaOH+CO2�TNa2CO3+H2O��
80 44
y 6.6g
$\frac{80}{y}$=$\frac{44}{6.6g}$��
y=12g��
��������������Һ��������������Ϊ��$\frac{12g}{100.0g}$��100%=12%��
����������������Һ��������������Ϊ12%��
���� ������̼����������ǡ����ȫ��Ӧʱ����������������Һ��������100.0g��Ҫע�����⣮

 �������Ͽ�ʱͬ��ѵ��ϵ�д�
�������Ͽ�ʱͬ��ѵ��ϵ�д� �������ͬ����ϰϵ�д�
�������ͬ����ϰϵ�д�| A�� | 50% | B�� | 80% | C�� | 12.5% | D�� | 20% |
| ѡ�� | ���ʣ����ʣ� | �������� |
| A | CO2��H2O�� | ������ͨ��ʢ�л��ĸ���� |
| B | Cu��CuO�� | ͨ���������������� |
| C | CO2��CO�� | ��ȼ |
| D | FeCl2��CuCl2�� | ����������м����ַ�Ӧ����� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | NaOH��Na2SO4��NaCl | B�� | NaCl��NaOH��Na2SO4 | ||
| C�� | NaCl��Na2SO4��NaOH | D�� | Na2SO4��NaCl��NaOH |
 �ֳ�ȡ̼������Ʒ6g�����Ƴ���Һ���������м���CaCl2��Һ����ӦʱCaCl2��Һ�������������ϵ��ͼ��ʾ�������������⣺
�ֳ�ȡ̼������Ʒ6g�����Ƴ���Һ���������м���CaCl2��Һ����ӦʱCaCl2��Һ�������������ϵ��ͼ��ʾ�������������⣺ ij�о���ѧϰС����Э����ʦ����ʵ����ʱ������һ����Ŷ�����������ƣ�Ϊ������������������������̽����ȡ����������Ʒ11.4g����ƿ�У�����38.6gˮ�����γ�����Һ�����ڵ�����ƽ�ϣ�����ƿ����εμ�14.6%��ϡ���ᣬ��ͼ����ʾ�����ȡ�������õ���ƿ�����ʵ�������ϡ����������ϵ����ͼ����ʾ������
ij�о���ѧϰС����Э����ʦ����ʵ����ʱ������һ����Ŷ�����������ƣ�Ϊ������������������������̽����ȡ����������Ʒ11.4g����ƿ�У�����38.6gˮ�����γ�����Һ�����ڵ�����ƽ�ϣ�����ƿ����εμ�14.6%��ϡ���ᣬ��ͼ����ʾ�����ȡ�������õ���ƿ�����ʵ�������ϡ����������ϵ����ͼ����ʾ������