��Ŀ����
4�� ij�о���ѧϰС����Э����ʦ����ʵ����ʱ������һ����Ŷ�����������ƣ�Ϊ������������������������̽����ȡ����������Ʒ11.4g����ƿ�У�����38.6gˮ�����γ�����Һ�����ڵ�����ƽ�ϣ�����ƿ����εμ�14.6%��ϡ���ᣬ��ͼ����ʾ�����ȡ�������õ���ƿ�����ʵ�������ϡ����������ϵ����ͼ����ʾ������
ij�о���ѧϰС����Э����ʦ����ʵ����ʱ������һ����Ŷ�����������ƣ�Ϊ������������������������̽����ȡ����������Ʒ11.4g����ƿ�У�����38.6gˮ�����γ�����Һ�����ڵ�����ƽ�ϣ�����ƿ����εμ�14.6%��ϡ���ᣬ��ͼ����ʾ�����ȡ�������õ���ƿ�����ʵ�������ϡ����������ϵ����ͼ����ʾ��������1������Ʒ���������Ƶ�������
��2����B��ʱ������Һ�����ʵ������������𰸱�����0.1%����
���� ���������������տ����еĶ�����̼����̼��ƶ����ʣ�����ʵ��������Ƶ���Һ�еμ����ᣬ���������������Ʒ�Ӧ�������Ȼ��ƺ�ˮ�������ܲ������壬���������ĵμ�ƿ�������������ӣ���ͼ���еĵ�һ��������ʾ��������������ȫ��Ӧ�����μӵ����Ὺʼ��̼��Ʒ�Ӧ������ͼ��ƿ������Ϊ100gʱʼ��̼��������ᷴӦ�����Ȼ��ƺ�ˮ��ͬʱ�ų����������̼�����������ĵμ�ƿ�������������������ͼ���еĵڶ������ߣ���̼�����ȫ��Ӧ�μӵ����������Ӧ��ƿ���������������ͼ���е��������ߣ��������������ƽη�Ӧ�Ļ�ѧ����ʽ������������������ɼ�����μӷ�Ӧ���������Ƶ�������̼��Ƶ�����Ϊ��Ʒ���������Ƶ��������Լ�̼�������������������з�����
��� �⣺��1���������ͼ�����֪����һ����ƿ���������ӵ�������������Ʒ���������Ʒ�Ӧ���������������һ�ε������������Ϊ100g-50g=50g
�������ᷴӦ��Ca��OH��2������Ϊx�������Ȼ���Ϊa
Ca��OH��2+2HCl=CaCl2+2H2O
74 73 111
x 50g��14.6% a
$\frac{74}{x}$=$\frac{73}{50g��14.6%}$=$\frac{111}{a}$
���x=7.4g��a=11.1g
���ڻ������̼��Ƶ�����Ϊ��11.4g-7.4g=4g��
��2��4g̼����������������Ϊy�����ɶ�����̼������Ϊz�������Ȼ���Ϊb��
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 73 111 44
4g y��14.6% a z
$\frac{100}{4g}$=$\frac{73}{y��14.6%}$=$\frac{44}{z}$=$\frac{111}{a}$
y=20g
z=1.76g
a=4.44g
y���ϵ�B������ʾ��ƿ�е���������=100g+20g-1.76g=118.24g��
B��ʱ������Һ�����ʵ���������Ϊ��$\frac{11.1g+4.44g}{118.24g}$��100%=13.1%��
�𣺣�1������Ʒ���������Ƶ�������7.4g��̼��Ƶ�������4g��
��2����B��ʱ������Һ�����ʵ���������Ϊ13.1%��
���� �������ʼ䷴Ӧ���Ⱥ��ϵ��������Ӧ������������������Ӱ�죬�ó���������Һ��������Ȼ����ݷ�Ӧ�Ļ�ѧ����ʽ����ɷ�������㣮

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| ���� | �� | ���� | ������̼ | ˮ | X |
| ��Ӧǰ����/g | 3.9 | 9.6 | 0 | 0 | 0 |
| ��Ӧ������/g | 0 | 0 | 6.6 | 2.7 | m |
| ʵ����� | ����ϡ���������/g | ʣ����������/g |
| 1 | 20 | 11 |
| 2 | 20 | 6 |
| 3 | 20 | 2.8 |
| 4 | 20 | n |
��2����Ʒ��̼��Ƶ�����������82.5%��
��3����Ӧ����ʱ���ɶ�����̼��������
| A�� | K2MnO4��MnO2 | B�� | KMnO4��MnO2 | ||
| C�� | KMnO4��K2MnO4��MnO2 | D�� | KMnO4��K2MnO4 |
| A�� |  �Թ����� | B�� |  ��ȡ��Һ��ƫ�� | C�� |  ��Ƥ������ | D�� |  ���ھƾ�ȼ�� |
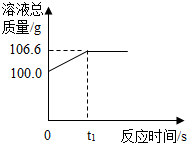 Ϊ�ⶨij������ʯ��������������������С��������ͬѧ��������һ����̼��10g������ʯ��Ʒ��ַ�Ӧ�����ʲ����뷴Ӧ�����������ɵ�������һ����������������Һ��ȫ���գ�����Һ�������뷴Ӧʱ��ı仯��ϵ��ͼ��
Ϊ�ⶨij������ʯ��������������������С��������ͬѧ��������һ����̼��10g������ʯ��Ʒ��ַ�Ӧ�����ʲ����뷴Ӧ�����������ɵ�������һ����������������Һ��ȫ���գ�����Һ�������뷴Ӧʱ��ı仯��ϵ��ͼ��