��Ŀ����
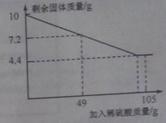
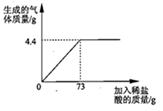 ijƷ�ƴ����к�������NaCl����ѧ��ȤС���ͬѧ����������ʵ��̽������ȡ12g��Ʒ�����ձ��У�����ϡ���������ٲ�������Ϊֹ�������Ƴ�����ϡ�����������ų����������Ĺ�ϵ��ͼ
ijƷ�ƴ����к�������NaCl����ѧ��ȤС���ͬѧ����������ʵ��̽������ȡ12g��Ʒ�����ձ��У�����ϡ���������ٲ�������Ϊֹ�������Ƴ�����ϡ�����������ų����������Ĺ�ϵ��ͼ
��1������ǡ����ȫ��Ӧʱ������CO2������Ϊ g
��2���������Ʒ�к����ʵ��������� �Ƕ��٣�������������һλС������ͬ��
�Ƕ��٣�������������һλС������ͬ��
��3�����㵱�����봿 ��ǡ����ȫ��Ӧʱ��������Һ���������������Ƕ��٣�
��ǡ����ȫ��Ӧʱ��������Һ���������������Ƕ��٣�
���𰸡���1��4.4
��2���⣺����Ʒ��̼���Ƶ�����Ϊx����ȫ��Ӧ�����Ȼ��Ƶ�����Ϊy
Na2CO3 + 2HCl === 2NaCl + H2O + CO2��
106 117 44
x y 4.4g
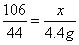
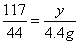
��ã�x=10.6g y=11.7g
������Ʒ���Ȼ��Ƶ�����Ϊ12g-1.06g=1.4g
�Ȼ��Ƶ���������Ϊ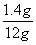 ��100��=11.7��
��100��=11.7��
��3����Ӧ����Һ������Ϊ73g+12g-4.4g=80.6g
1.4g+11.7g=13.1g
����������Һ��������������Ϊ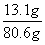 ��100��=16.3��
��100��=16.3��
����Ʒ�������Ȼ��Ƶ���������Ϊ11.7��������ǡ����ȫ��Ӧʱ��������Һ��������������Ϊ16.3����
����������ͼ��ֱ�ӻ�ȡ��ȫ��Ӧ���ɶ�����̼������Ϊ4.4g�����ö�����̼�������ɼ����̼���Ƶ��������ٸ�����Ʒ��������������Ȼ��Ƶ���������������غ㶨�ɿɼ������Ӧ��������Һ���������������ɶ�����̼�������ɼ������Ӧ�����Ȼ��Ƶ����������������Һ����������������

 ״Ԫ��ȫ��ͻ�Ƶ�����ϵ�д�
״Ԫ��ȫ��ͻ�Ƶ�����ϵ�д� ֱͨ������У�ܲ��¿�ֱͨ��Уϵ�д�
ֱͨ������У�ܲ��¿�ֱͨ��Уϵ�д�

 ________��
________��